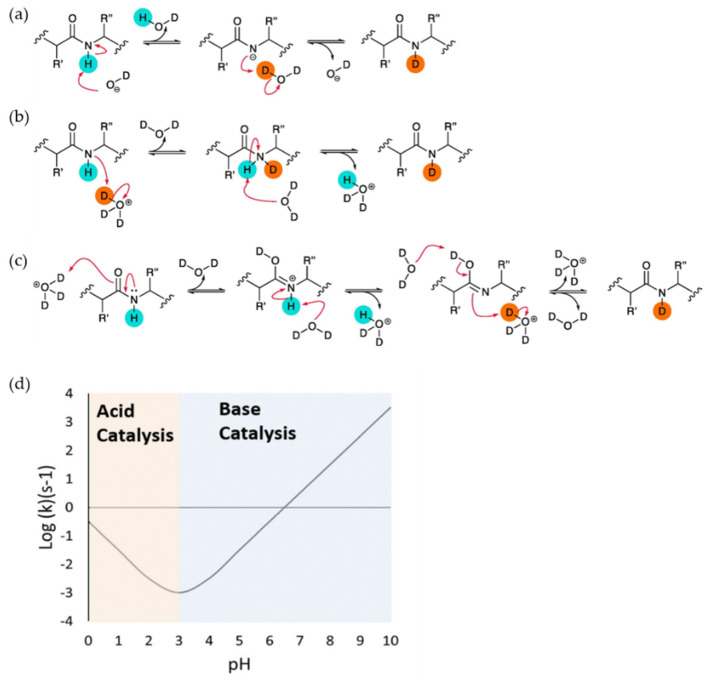
Figure 1.
Schematic of acid and base catalysis of peptide group protons in solution during H-D exchange and the dependence of intrinsic rate of exchange (kch) on the pH of solution. (a) Base catalysis (b) Acid catalysis through the protonation of peptide group N atom. (c) Acid catalysis through protonation of peptide group O atom. Reproduced with permission from Journal Methods@ 2018. (d) The dependence of intrinsic rate of exchange (kch) of poly-DL-alanine on the pH showing that the minimum exchange is at pH 2.5–3. Acid catalysis is dominant below the pH and base catalysis is dominant above the pH. Reproduced with permission from the journal Analyst (© 2017).