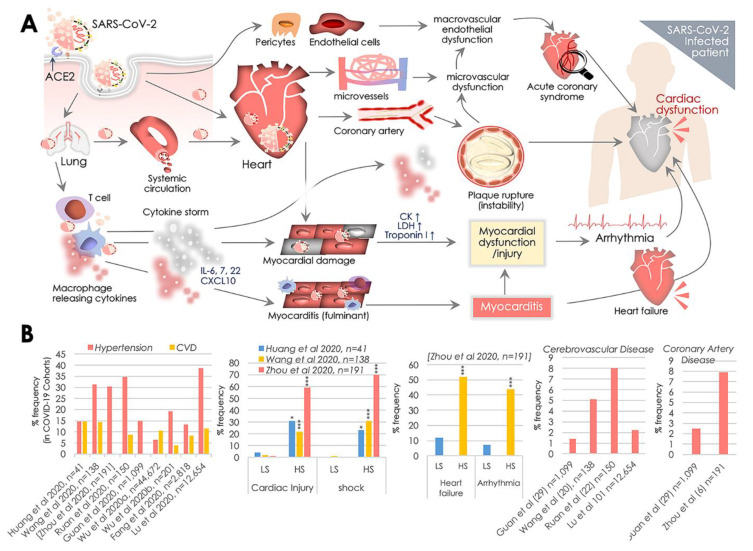
Figure 1.
SARS-CoV-2, angiotensin converting enzyme 2 (ACE2), and cardiovascular complications. (A) Transmembrane ACE2 receptor facilitates SARS-CoV-2 entry to host cell primarily in the lungs, and then the vascular system, postulating cardiovascular complications by causing inflammation and myocardial dysfunction. SARS-CoV-2 access to the systemic circulation via the lungs potentiates heart infection, while its direct infection of associated pericytes and endothelial cells may cause vascular endothelial dysfunction. Cardiac SARS-CoV-2 infection causes micro-vessel dysfunction, and elevated immunoreactivity disrupts atherosclerotic plaques leading to the progression of the acute coronary syndromes. SARS-CoV-2 infection of alveolar pneumocytes (type II) cells progressively develops the systemic inflammation and elevated immunoreactivity that eventually produces the ‘cytokine storm’, marked by elevated IL-6, IL-7, IL-22, and CXCL10 cytokine levels. It potentiates T-cell and macrophage activation infiltrating infected myocardial tissues and may produce severe cardiac damage and myocarditis, leading to heart failure. Cytokine storm may further increase damage of cardiac monocytes causing myocardial dysfunction and subsequent development of arrhythmia. These events cumulatively produce cardiac dysfunction. (B) Manifestation (%) of cardiovascular complications in hospitalized COVID-19 patients reported in key clinical studies exhibiting comorbidities including hypertension, cardiovascular disease (CVD), cerebrovascular disease, coronary artery disease and rate of cardiac injury, shock, heart failure, and arrhythmia in low (LS), and high severity (HS) patient groups. p values indicate *** (<0.001), ** (<0.01), and * (<0.05) statistical significance.