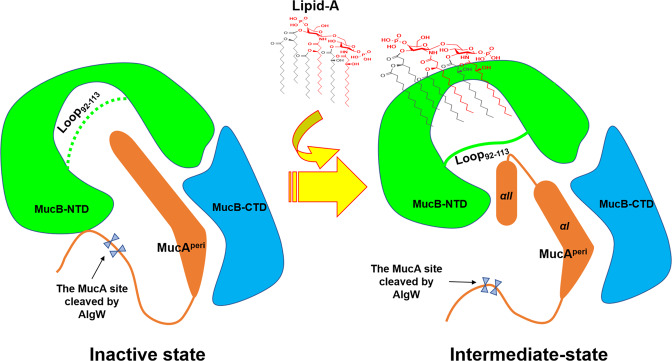
Fig. 6. The model of MucA–MucB conformational conversions in response to lipid signals.
a Inactive state. MucB protects MucA from intramembrane proteolysis by AlgW proteases. The MucAperi, the NTD and CTD of MucB are colored in orange, green, and blue. The loop92–113 is represented by a green dotted line, which means that the loop is foled into the inner cavity of MucB, and forms a steric hindrance to prevent the binding of amphiphilic effectors like lipid-A. The cleavage site of MucA cleaved by AlgW is protected by MucB. b Intermediate state. Lipid binding induces structural changes in MucA–MucB and exposes the degradation site of MucA, these structural changes include: 1. The interaction between the NTD and CTD of MucB becomes a relatively weak van der Waals contacts mediated by water. 2. The loop92–113 flips to the surface of MucB, forming a steric hindrance and inducing the MucA αII to insert into the inner cavity of MucB. 3. The cleavage site of MucA cleaved by AlgW was exposed to solvent, which initiates the MucA proteolysis process.