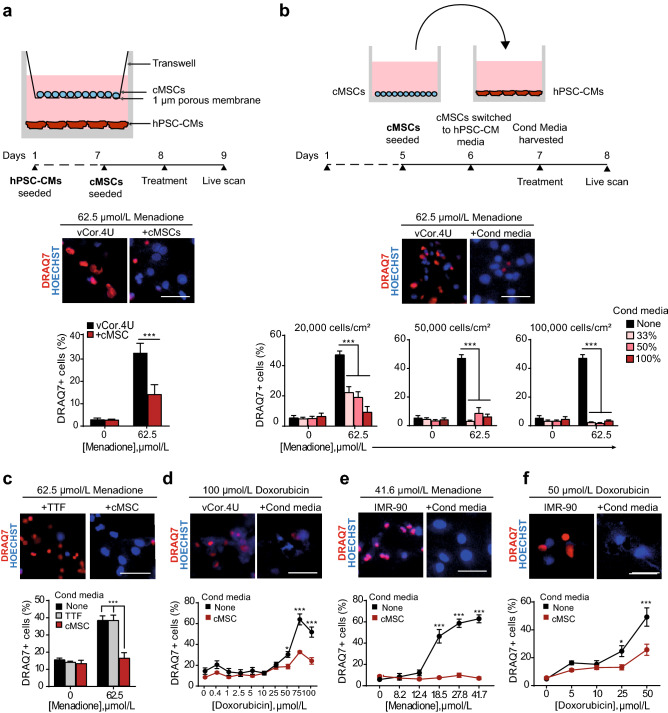
Figure 1.
Mouse cMSC secretome suppresses cell death in human cardiomyocytes from pluripotent stem cells. (a) cMSC protect vCor.4U human ventricular myocytes from lethal oxidative stress in trans-well co-culture. Above, schematic representation and timeline. Middle, representative images of nuclear DRAQ7 staining after a menadione challenge. Below, bar graph of DRAQ7 uptake; n = 6. cMSC were prospectively sorted using Sca1 and the SP phenotype, and were 90% PDGFRα+. (b) cMSC-conditioned media protect vCor.4U human ventricular myocytes from lethal oxidative stress. Above, schematic representation and timeline. Middle, representative images as in (a). For the cultures illustrated, cMSC were seeded at 100,000 cells/cm2 and conditioned media used at a concentration of 50%. Below, bar graphs of DRAQ7 uptake, at the indicated cMSC seeding densities and media concentrations; n = 9. (c,d) Specificity and generality of protection in vCor.4U human ventricular myocytes. Above, representative images. Below, bar graphs of DRAQ7 uptake; n = 9. (c) Lack of protection from menadione by tail tip fibroblast-conditioned medium. (d) Protection from doxorubicin by cMSC-conditioned media. (e,f) cMSC-conditioned media was tested on IMR-90 hPSC-CMs, an independent human cardiomyocyte line. (e) Representative images and bar graph of DRAQ7 uptake after menadione. n = 12. (f) Representative images and bar graph of DRAQ7 uptake after doxorubicin; n = 9. For all panels: scale bar, 50 μm; data are shown as the mean ± SEM; *p < 0.05; ***p < 0.0001.