Fig. 2.
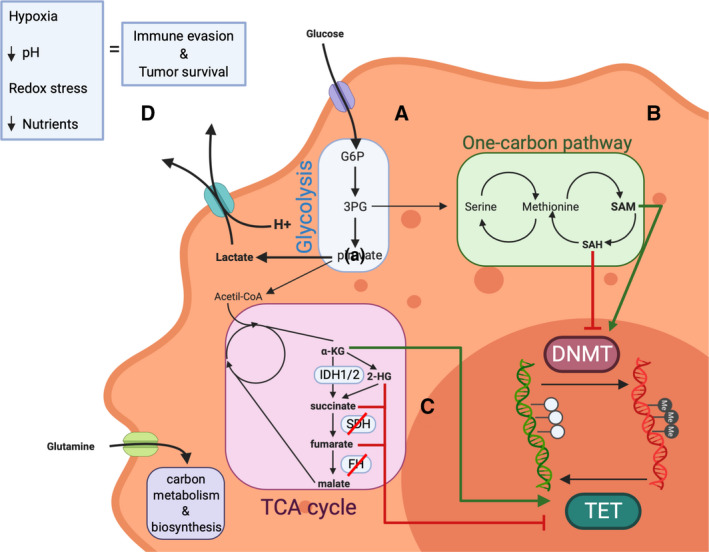
Cellular regulation of DNA methylation. DNA methylation influences neoplastic cell's metabolism and vice versa. (A) Glycolysis is regulated by, among others, the HIF1 pathway. A crucial TSG of this pathway, VHL, has been shown to be epigenetically silenced in hematological malignancies. See text for more details. (B) SAM is the substrate needed by DNMTs in order to methylate the DNA, and it is one of the limiting aspects that favors DNMT impairment in tumors. SAM is then converted to SAH, which usually accumulates and acts as an inhibitor of the process in the normal product‐negative regulation of the enzyme function. See text for more details. (C) Although the tumor cell prefers to transform glucose into lactate to rapidly obtain ATP, intermediate metabolites and redox power, TCA cycle intermediates play an important role in methylation as several of them act upon TET demethylases. TET enzymes use α‐KG as a substrate to actively demethylate DNA, and, as SAM, it is rather limited in the tumor cell. α‐KG can be transformed into 2‐HG by mutated forms of IDH1 or IDH2, which acts as a competitor of α‐KG and impairs TET function. SDH and FH might be silenced in hematological malignancies, which originates an accumulation of succinate and fumarate, which together with 2‐HG act as TET inhibitors in the cell. (D) Due to the increase in nutrient uptake, hypoxic conditions, redox stress, and environment acidification, the tumor cell creates an environment, which enhances tumor survival while it dampens immune cell activation, that is, favoring macrophage M2 polarization or Treg phenotype. 3PG, 3‐phosphoglycerate; G6P, glucose‐6‐phosphate; α‐KG, α‐ketoglutarate.
