Abstract
Acetylsalicylic acid, also known as aspirin, is often used in clinical antipyretic, analgesic and antiplatelet therapy. Aspirin can cause numerous side effects in the gastrointestinal (GI) tract, ranging from unpleasant GI symptoms without gastric mucosal lesions to ulcer bleeding and even death. However, recent studies have found that aspirin can significantly prevent GI tumors. Despite impressive advances in cancer research, screening and treatment options, GI tumors remain a leading cause of death worldwide. Prevention is a far better option than treatment for tumors. Therefore, the present review assesses the pros and cons of aspirin on the GI tract and, on this the basis, the appropriate dose of aspirin to protect it.
Keywords: aspirin, effect, gastrointestinal cancer, gastrointestinal side effects, gastrointestinal benefit
1. Introduction
Cancer is a major cause of death and one of the most important global obstacles to improving life expectancy in the 21st century (1). Despite significant advances in cancer research, screening and treatment programs, gastrointestinal (GI) tumors have a high morbidity and mortality rate worldwide; according to the 2018 global cancer report, the mortality rate of colorectal cancer was 9.2%, that of gastric cancer was 8.2% and that of liver cancer was 8.2%, which account for the top 5 cancer mortality rates in the world (2). The high incidence of cancer in the digestive system, especially gastric, esophageal and colorectal cancer (CRC), is a major health burden in Asia, particularly in East Asia. Asia had the highest rate of CRC cases per 100,000 population (51.8%) and the highest mortality rate (52.4%) in 2018 (3). Additionally, >727,000 cases of gastric cancer were diagnosed in Asia in 2008, accounting for 11.9% of all confirmed cancer cases (4). Morbidity and mortality rates are also higher in East Asia than in other parts of the continent (4). China has the highest mortality rate of gastric cancer (30.1 per 100,000), followed by Japan (20.5 per 100,000) and South Korea (13.8 per 100,000) (4).
Since Bayer AG distributed acetylsalicylic acid as Aspirin™ in 1899, the drug has cornered the non-steroidal anti-inflammatory drug (NSAID) market, and with time, aspirin has become a generic name (5). Recently, aspirin has received increasing research attention. The effectiveness of low-dose aspirin (LDA) in preventing ischemic cardiovascular events has been confirmed (6–8). There is increasing evidence to support the role that aspirin may play in chemical protection, especially in patients without cardiovascular disease (CVD) (9–11). Using aspirin can reduce mortality rates and the incidence of GI tumors (12). However, aspirin can cause a range of GI side effects; aspirin taken in large doses has anti-inflammatory effects similar to those caused by non-selective NSAIDs in the GI tract (13). LDA is now widely used for cardiovascular prevention, but even at these very low doses it is still associated with upper and lower GI damage (14). In the upper GI tract, aspirin causes a series of adverse GI events, ranging from asymptomatic lesions to serious complications, such as peptic ulcer bleeding or even death (15). The link between aspirin and upper GI damage has been established, but the impact of aspirin on the lower GI tract remains unclear, although evidence has been mounting over the past few years (15,16).
The present review focuses on the advances made in understanding the benefits and the risks of long-term use of aspirin on the GI tract. To provide evidence for the rational use of aspirin in clinical practice, the appropriate dose of aspirin to protect the GI tract is analyzed and summarized.
2. Pros
The main benefit of aspirin in the GI tract is manifested in the chemoprophylaxis of GI adenoma and numerous types of cancer, such as CRC, gastric cancer and familial adenomatous polyposis (FAP). The main pros of aspirin, as will now be described, are also summarized in Table I. Additionally, the present review identified that the chemoprophylaxis dose of aspirin varied among studies. Therefore, the appropriate preventive doses of aspirin used in different studies are summarized in Table I.
Table I.
Association of the benefits of aspirin in the GI tract and the dose at which aspirin is effective from recent trials and studies.
| A, Colorectal adenomas | |||||
|---|---|---|---|---|---|
| First author, year | Study type | No. of cases | Dose | Results | (Refs.) |
| Sandler et al, 2003 | RCT | 635 | 325 mg/day | Reduced the number of adenomas and delayed the development of adenomas in the patients who had CRC history | (20) |
| Hull et al, 2018 | RCT | 709 | 300 mg/day | Reduced the total number of colorectal adenomas per participant | (117) |
| Baron et al, 2003 | RCT | 1,121 | 81–325 mg/day | Had a moderate chemopreventive effect | (21) |
| B, Familial adenomatous polyposis | |||||
| First author, year | Study type | No. of cases | Dose | Results | (Refs.) |
| Ishikawa et al, 2013 | RCT | 34 | 100 mg/day | Polyp size tended to be smaller, especially in the subjects with a mean baseline polyp diameter <2 mm | (27) |
| Burn et al, 2011 | RCT | 133 | 600 mg/day | No reduction in polyps | (28) |
| C, CRC | |||||
| First author, year | Study type | No. of cases | Dose | Results | (Refs.) |
| Cole et al, 2009 | Meta-analysis of RCTs | 3,000 | 325 mg/day | Decreased the relative risk of adenoma | (118) |
| Cook et al, 2013 | RCT | 39,876 | 100 mg/day | Decreased the risk of CRC | (119) |
| Cea Soriano et al, 2017 | Cohort study | 263,482 | LDA (the exact dose was not specified) | Reduced the incidence of CRC | (29) |
| Kuan et al, 2019 | Cohort study | 344,777 | ≥28 cumulative defined daily doses | Reduced the CRC risk in patients with type 2 diabetes mellitus | (120) |
| Flossman and Rothwell, 2007 | Review of 19 case-control studies and 11 cohort studies | 1,136,110 | ≥300 mg/day | Reduced the incidence of CRC | (34) |
| Rothwell et al, 2010 | Cohort study | 14,033 | ≥75 mg/day | Reduced incidence and mortality due to CRC | (35) |
| Cao et al, 2016 | Cohort study | 135,965 | 0.5–1.5 standard aspirin tablets weekly | Reduced the incidence of CRC and had a dose-dependent effect of aspirin | (30) |
| D, Gastric cancer | |||||
| First author, year | Study type | No. of cases | Dose | Results | (Refs.) |
| Li et al, 2016 | Experimental study | 20 mice | 250 mg/kg | Aspirin inhibited the proliferation and migration of gastric cancer cells in mice | (36) |
| Rothwell et al, 2012 | RCT | 17,285 | ≥75 mg/day | Reduced the incidence of gastric cancer and CRC and reduced the risk of distant metastasis | (121) |
| Rothwell et al, 2011 | RCT | 25,570 | ≥75 mg/day | Reduced deaths caused by gastric cancer | (39) |
| Thun et al, 1993 | Meta-analysis of RCTs | 635,031 | Unclear | Reduced risk of fatal cancer of the esophagus, stomach, colon and rectum | (38) |
| Cao et al, 2016 | Cohort study | 135,965 | 0.5–1.5 standard aspirin tablets weekly | Reduced risk of gastric cancer | (30) |
| E, Esophageal cancer | |||||
| First author, year | Study type | No. of cases | Dose | Results | (Refs.) |
| González-Pérez et al, 2003 | Meta-analysis of RCTs | – | Lack of specific dose | Reduced the risk of esophageal cancer and gastric cancer | (42) |
| Liu et al, 2009 | RCT | 1,716 | 25–50 mg/day | Reduced the risk of both esophageal cancer and esophageal squamous cell carcinoma | (43) |
| Vaughan et al, 2005 | Prospective cohort study | 350 | ≥75 mg/day | Reduced the risk of neoplastic progression in Barrett's esophagus | (41) |
| Farrow et al, 1998 | Case-control study | 695 | No specific dose was indicated | Reduced the risk of cancers of the esophagus and stomach | (40) |
| Bosetti et al, 2012 | Review comprising of case-control and cohort studies | – | Unclear | Reduced the risk of esophageal cancer and gastric cancer | (46) |
| Spence et al, 2018 | Case-control study | 8,487 | 75 mg/day | Long-term aspirin was not associated with cancer-specific mortality after diagnosis of esophageal or gastric cancer | (48) |
| F, Other GI tumors | |||||
| First author, year | Study type | No. of cases | Dose | Results | (Refs.) |
| Rothwell et al, 2011 | RCT | 25,570 | 75 mg/day | Reduced the mortality of pancreatic cancer | (39) |
| Petrick et al, 2015 | Case-control study | 1,084,133 | ≤163 mg/day | Prevented liver cancer (including hepatocellular and intrahepatic cholangiocarcinoma) | (52) |
| Choi et al, 2019 | Case-control study | 4,962 | LDA but the exact dose remains unclear | Reduced risk of pancreatic ductal adenocarcinoma incidence for patients with risk factors | (49) |
CRC, colorectal cancer; GI, gastrointestinal; RCT, randomized control trial; LDA, low-dose aspirin.
Aspirin and colorectal adenomas
Adenomas are the most common polyps of the large intestine and are a precursor to most CRC (17). The epidemiology of adenomas and CRC is very similar, hence preventing adenomas can also prevent CRC (18,19). Sandler et al (20), found that taking 325 mg aspirin daily reduced the risk of adenomas and delayed progression in individuals with a CRC history by 10%. However, the aforementioned study contain bias, as it selected only high-risk patients. Baron et al (21), conducted another study in patients who had not previously ha5d CRC and found that 81 mg aspirin daily reduced the risk of recurrent adenomas by 19% in patients with a recent history of adenomas. In the group administered 81 mg aspirin daily, there was a >40% reduction in the risk of advanced disease.
FAP is a special type of colonic polyp (22). FAP is characterized by the development of multiple (>100) colorectal adenomas throughout the colorectum (23). It is an uncommon autosomal dominant genetic disease characterized by numerous adenomas in the large intestine (22). Half of the patients with FAP are reported to develop adenocarcinoma by the age of 40 years (24). Prophylactic colectomy or proctocolectomy is usually performed between ages 15 and 25 years (25). However, this type of intervention can cause severe diarrhea in some patients, resulting in a dramatic decline in quality of life. Therefore, research on chemoprophylaxis is being conducted in the hope of delaying surgery, or in patients with fewer polyps, to help avoid surgery altogether using a combination of chemoprevention and endoscopic resection (26).
To the best of our knowledge, there are few reports on the effect of aspirin on FAP, but several studies have reported that aspirin can lessen the number of adenomas and delay the development of the disease. In a double-blind, randomized study where 34 patients with FAP were treated with LDA (100 mg/day) or placebo post-colectomy, the size of colorectal polyps in patients with FAP tended to be smaller when treated with LDA (27). In addition, the diameter and number of polyps were markedly reduced (aspirin:placebo group response ratio, 2.33:1) and polyp height also decreased (aspirin:placebo group response ratio, 2:1) (27). These results demonstrated that aspirin may have an impact on the relatively early stage of CRC development. However, the aforementioned study had limitations such as a small sample size and limited fields of polyp counting, which may have hampered the detection of responses. Another study by Burn et al (28), treated patients with FAP with aspirin (600 mg/day) and/or placebo for 1–12 years and reported no side effects from aspirin, but also no reduction in polyps. The differences between these 2 studies may be due to differences in cohorts of patients and the dose of aspirin given.
Current research has demonstrated that aspirin does indeed affect adenomas; aspirin prevented the growth of adenomatous polyps leading to the regression of existing polyps in several randomized trials of patients with FAP (27). However, the degree of effect of aspirin dose on adenoma is still controversial. As there are few studies on the effect of aspirin on FAP, which are hampered by the low incidence of the disease (1 in 8,300 at birth) (22) and small patient sample size, further studies are required to explore and verify their findings.
Aspirin and CRC
CRC ranked third in terms of incidence, but second in terms of mortality rate worldwide in 2018 (2). In patients with CRC with or without known CVD, there is little debate about the preventive effects of long-term aspirin use. Considerable experimental evidence supports this view.
Numerous studies have demonstrated that regular aspirin use does prevent or reduce CRC mortality rates. The effects of aspirin may be related to the duration of use. Cea Soriano et al (29) used The Health Improvement Network to estimate the incidence of CRC in individuals without CVD and demonstrated that regular LDA use is associated with a reduction in the incidence of CRC in individuals without CVD. In addition, incidence rate ratios demonstrated that starting LDA at an age of 60–69 or 70–79 years significantly reduced the CRC risk. However, starting LDA at 80–89 years did not reduce the risk of CRC. A study that used data from two ongoing prospective studies, the Nurses' Health Study and the Health Professionals Follow-up Study, which contained follow-up data for patients with CRC up to 32 years (30), discovered a dose-dependent effect of aspirin on CRC, appearing at 0.5–1.5 standard aspirin tablets weekly or the equivalent of a daily dose of LDA. A combined analysis of dose and duration demonstrated that the apparent benefit of aspirin use for GI cancer and CRC comes from taking 0.5–1.5 standard aspirin tablets weekly (30). Regular aspirin use could prevent 33 CRCs/100,000 person-years among individuals who are older >50 years (30).
The majority of studies have demonstrated a decreasing risk of CRC or adenomas when aspirin therapy is stopped (31–33). There are different opinions about the duration of aspirin use. Some studies have demonstrated that this effect does not appear until 5–10 years later, which is a considerably delayed effect (34,35).
Aspirin and gastric cancer
Gastric cancer was the fifth most commonly diagnosed cancer in the world and the third leading cause of cancer death in 2018 (2). Among men, it is the most common cancer in several western Asian countries, including Iran, Turkmenistan and Kyrgyzstan, and is also the main cause of cancer death (2).
A previous study using animal experiments suggested that aspirin may inhibit not only the growth of gastric cancer but also the migration of cancer cells (36). Li et al (36) used p53−/− mice (n=20) that were randomly divided into 2 groups to understand the effects of aspirin on gastric cancer. The aforementioned study demonstrated that the proliferation capacity of tumor cells in the experimental group (250 mg/kg aspirin daily added to food) was significantly reduced compared with that of the control group, and the number of cells was also markedly reduced.
To the best of our knowledge, there are few specific studies on the effects of aspirin on gastric cancer. The extent of the impact of aspirin on gastric cancer seems to be smaller than that on CRC and colorectal adenoma; in addition, the data are less comprehensive and more variable. However, it can be concluded from multi-factor analysis in numerous studies that aspirin can inhibit the growth of gastric cancer. Two cohort studies have found that there was a 41% reduction in mortality in patients with gastric cancer when aspirin was taken (37,38). In a study involving several randomized controlled trials (RCTs), reported deaths decreased by 31% in patients with gastric cancer who took aspirin (P=0.11), and most significant decreases of up to 58% (P=0.007) were observed after 10 years of use (39).
Aspirin and esophageal cancer
The incidence and mortality of esophageal cancer were ranked seventh and sixth among all types of cancer, respectively, in the 2018 global cancer statistics (2). Due to the poor prognosis of esophageal cancer, treatment options have been highlighted in this section of the review.
The effect of long-term aspirin use in Barrett's esophageal cancer has been studied by several clinical trials and population-based observational studies, suggesting that aspirin may slow down its progression to both squamous carcinoma and adenocarcinoma (40–42). Another three independent studies reported that in patients with esophageal cancer, aspirin had a remarkable protective effect on all-cause mortality (43–45). In all three studies, these effects were limited to patients with esophageal cancer (44) and esophageal squamous cell carcinoma (43), and in the most recent study (45), when methods to reduce immortal time bias were employed, the effects were attenuated. A meta-analysis indicated that aspirin does have a protective effect on esophageal cancer (including gastric cardia cancer), regardless of squamous cell or adenocarcinoma type (46). In addition, in a recent study, in patients with Barrett's esophageal cancer who did not have a history of NSAID use, aspirin showed a significant protective effect (47).
However, opposing results were found by a study that included two large independent population-based cohorts in the UK (48). The study aforementioned revealed that long-term aspirin use (75 mg/day) was not associated with cancer-specific mortality after the diagnosis of esophageal or gastric cancer (48). These differences may be due to the aforementioned study investigating the effects of using aspirin on cancer after a diagnosis of gastric or esophageal cancer, whereas in other studies, patients were taking aspirin prior to esophageal or gastric cancer diagnosis.
Aspirin and other GI tumors
Aspirin can reduce cancer-related mortality in all solid cancer types. One study found that this was mainly due to fewer deaths after 5 years, including significant reductions in pancreatic cancer deaths (39). In the aforementioned study, aspirin had a significant effect on pancreatic cancer death only after >7.5 years of planned treatment [hazard ratio (HR), 0.28; 95% confidence interval (CI) 0.08–1.00; P=0.04]. Another study found that aspirin use was associated with a reduced risk of pancreatic ductal adenocarcinoma incidence in patients with risk factors (OR, 0.48; 95% CI, 0.31–0.67; P<0.001) (49).
Previous studies have suggested that aspirin may have the potential to prevent liver cancer [including hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC)] (50,51). A prospective cohort study based on data from 1,084,133 individuals (HCC, n=679; ICC, n=225) from 10 counties in the United States found that aspirin users who took the drug once a day (>5 years; ≤163 mg) had a 32% reduction in the risk of liver cancer (52). In addition, aspirin use reduced ICC risk by 36% in men, but not in women. For some patients with liver cancer who are at higher risk of bleeding (due to, for example, cirrhosis of the liver and portal hypertension with thrombocytopenia), aspirin should be avoided due to the risk of GI bleeding and renal failure (53). Therefore, the use of aspirin in these patients remains an important matter and should be investigated further.
3. Possible mechanisms of the anticancer action of aspirin
The process by which normal cells turn into cancer cells is very complex and despite extensive study, the mechanisms by which aspirin may affect carcinogenesis remain unknown. However, in the early stages of cancer development, a common feature is that cells no longer respond to anti-proliferation and -differentiation signals, instead automatically producing signals to promote growth (54). The role of aspirin may be related to these processes.
One of the mechanisms involved in the anticancer activity of aspirin is commonly thought to be its ability to inhibit the activity of cyclooxygenase (COX) (55,56). COX-2 is strongly and rapidly induced in response to growth factors, cytokines, endotoxins and inflammation, and involves cell proliferation and the promotion of tumor development (57). As aspirin is a potent inhibitor of COX-2, it can reduce the production of prostaglandins and other inflammatory mediators (58). The deleterious effects of prostaglandins include promoting cell survival, stimulating cell proliferation and promoting angiogenesis, which can increase cancer metastasis (59,60).
However, the chemoprophylaxis of aspirin cannot be explained by inhibiting prostaglandin synthesis only, since some NSAIDs are still resistant to proliferation in cells that have no COX activity (61). COX-independent evidence has also been found in recent years, particularly in high-dose aspirin (31,62–65), which has been reported to induce apoptosis through the inhibition of activation of nuclear factor κB (NF-κB), the upregulation of tumor suppressor genes, such as TP53, CDKN1A and BAX, and the downregulation of antiapoptotic genes such as BCL-2 (55). Maintaining homeostasis requires apoptosis, or programmed cell death, to maintain a dynamic balance of the total number of cells in a tissue (66). Previous studies (67,68) reported reduced microsatellite instability and enhanced apoptosis in mismatch repair (MMR)-deficient cells exposed to aspirin, and suggested that aspirin may induce genetic selection for microsatellite stability in a subset of MMR-deficient cells. Aspirin may delete those aberrant stem cells most likely to progress rapidly to cancer (69). Recently, autophagy has been found to play a role in the chemoprevention of cancer by aspirin. It has been reported that LDA can induce autophagic death and inhibit the proliferation of various tumor cells, including HCC and CRC cells (70,71). Adenosine monophosphate-activated protein kinase (AMPK) can inhibit the growth and proliferation of tumor cells by affecting autophagy and inhibiting glycolysis. Recent studies have demonstrated that aspirin can promote the autophagy and apoptosis of tumor cells by activating AMPK (72,73). Aspirin can activate AMPK by the allosteric effect and by inhibiting the dephosphorylation of AMPKα at the Thr 172 site; AMPKα is an important activator of AMPK (74).
In addition, it has been reported that aspirin can stop or delay the growth and mutation of tumor cells instead of killing them directly. Aspirin can reduce the abnormal accumulation of genes in cancer tissues (75). Aspirin may also play a role in the DNA mismatch-repair system, Wnt signaling, NF-κB signaling and polyamine metabolism (76,77). Despite several recognized mechanisms of action for the anticancer effect of aspirin, the exact mechanism remains unknown.
4. Cons
The main cons of aspirin, as will now be described, are also summarized in Table II.
Table II.
Side effects of aspirin on the GI tract and related preventive measures.
| Region affected | Main risk factors | Dose | Adverse drug reaction | Precaution |
|---|---|---|---|---|
| Upper GI tract | H. pylori infection and advanced age (>70) | LDA but the exact dose remains unclear. | UGSs, peptic ulcers and bleeding, perforation | PPI prophylaxis may be considered for patients with related risk factors (Fig. 1) |
| Lower GI tract | Older patients with a great number of comorbidities (122,123) | Even LDA was associated with damage (124) and increased doses can cause more severe bleeding (125) | Lower GI bleeding | Some studies have evaluated the efficacy of probiotics in preventing lower GI injury among patients treated with LDA, but there is insufficient evidence to recommend a specific probiotic (126,127). |
| Lower GI tract | Obesity, hypertension, anticoagulants, diabetes mellitus, and ischemic heart disease (16) | There is no dose-dependent effect but a greater risk with the increasing duration of regular aspirin use (98) | Diverticular bleeding and diverticulitis | There is no established prophylaxis for diverticular bleeding and diverticulitis. |
| Liver | Patients with hepatic dysfunction (99) | Dose-dependent, 75–300 mg/day does not result in liver damage | Hepatic injury (acute fulminant liver failure or with no symptoms but with the biochemical and histological characteristics of chronic active hepatitis) | Avoid large doses of aspirin. |
GI, gastrointestinal; LDA, low-dose aspirin; PPI, proton pump inhibitor; H. pylori, Helicobacter pylori.
Aspirin and upper GI adverse effects
Upper GI symptoms (UGSs)
UGSs are the leading cause of discontinuation in patients treated with aspirin (78,79). Aspirin is one of the most common causal agents of medication hangover leading to hospitalization (80). UGSs are common but often overlooked. In particular, short-term use of aspirin for the treatment of pain, colds or fever did not show serious adverse events, but increased the risk of mild GI illness (81). A study known as ‘The UGLA survey’ demonstrated that the most frequent GI reaction among individuals using LDA was gastroesophageal reflux, followed by gastric burning and acid regurgitations (82). Most of the subjects (72%) reported that UGSs negatively affected their daily lives, even at low doses of aspirin, and damaged their quality of life to varying degrees (82). UGSs were present in one-fifth of patients taking LDA, resulting in poor compliance. In addition, a history of dyspepsia led to the occurrence of UGSs associated with LDA (82).
Peptic ulcers and bleeding
Ulcers are lesions that extend throughout the thickness of the mucosa and into the submucosa or deeper layers (83). Evidence of aspirin-induced peptic ulcers, particularly stomach ulcers began to emerge in the 1960s. Small ulcers are common even at the low doses of aspirin currently used for cardiovascular protection (78,84). One trial involving patients from four different countries who took 75–325 mg of aspirin daily for baseline endoscopic evaluation found that the annualized incidence of new ulcers was 28%, and the majority of ulcers were in the stomach (78). Another trial found similar rates in LDA users (84). Helicobacter pylori (H. pylori) infection and advanced age (>70 years) significantly increased the risk of ulcers (78). Other studies demonstrated that in NSAID users, H. pylori infection increased the risk of uncomplicated peptic ulcer by 2–3.5 times and GI bleeding by 2–2.5 times (85–87). Complications of peptic ulcers, especially the risk of bleeding, are associated with aspirin (88). A review analyzing studies published between 1946 and 2015 on the risk between long-term LDA and GI bleeding found that the incidence of GI bleeding with LDA was 0.48–3.64 cases per 1,000 person-years (89). A recent study involving healthy elderly individuals who did not have known CDV found that aspirin was associated with a notably higher risk of bleeding (90). The main bleeding events involved the upper GI tract and intracranial hemorrhage. A review of risk analysis of prophylactic use of aspirin in the general population demonstrated a significant increase in bleeding and ulcer events at 70 years of age, with long-term aspirin use reducing the risk of aspirin-related excessive bleeding (87).
GI perforation
Perforation is also a serious GI side effect of aspirin. A case-control study found that in the general population, LDA used to prevent CVD conferred a two-fold increased risk of upper GI perforation (91). Aspirin had the same effect on the gastric and duodenal sites. The aforementioned study reported that patients have the greatest risk of serious GI complications in the first 2 months after starting aspirin, following which, the risk falls and reaches a plateau at ~6 months. Aspirin is the most important independent risk factor for perforation of the upper and lower alimentary tract, and a history of smoking, alcohol consumption, arthritis or peptic ulcers increases the risk of perforation (92).
Aspirin and lower GI adverse effects
The association between LDA use and upper GI injuries has been well established, but effects of aspirin on the lower GI tract remain unclear, despite increasing evidence in recent years. A Japanese study reported that LDA (100 mg) significantly increased the risk of lower GI bleeding, but the number of bleeding cases (n=44) was relatively small (93). In a Spanish case-control study (>1,000 bleeding observations), LDA increased the risk of lower GI bleeding (including bleeding from the small and large intestine) by 2.7 times compared with no aspirin use (94). A study in Japan reported that CVD patients prescribed LDA for >1 year have a high risk of hemorrhage, but few cases (5/701) of bleeding were observed, so the CI was large and the finding not statistically significant (95).
In addition, the association between LDA and the development of diverticular bleeding and diverticulitis have been evaluated by several studies (16,96,97). In Japan, recent prospective research on diverticular disease has assessed the efficacy of LDA and found a significant association with diverticular bleeding (97). A study by health professionals demonstrated an increase in the risk of diverticular bleeding with LDA (2–5.9 tablets of 325 mg per week; multivariate HR, 2.32; 95% CI, 1.34–4.02) compared with no aspirin use (98).
Aspirin and hepatic injury
Hepatotoxicity induced by NSAIDs is a rare but potentially fatal complication that usually occurs within 12 weeks of initiation of treatment; it can occur in all NSAIDs, but seems to be more common when diclofenac and sulindac are used (99). Although there are few reports on the association between aspirin and hepatotoxicity, the side effect of hepatotoxicity in patients with hepatic dysfunction should be noted. The patients may present with acute fulminant liver failure or with no symptoms, but with the biochemical and histological characteristics of chronic active hepatitis (99). The hepatotoxicity of aspirin is dose-dependent and generally does not result in liver damage (75-300 mg/day) unless the full anti-inflammatory doses are used (99).
5. Conclusions
In general, the benefits of aspirin to the GI tract are mainly reflected in cancer prevention. Aspirin can reduce the incidence of gastric cancer, CRC, colorectal adenoma, live cancer and pancreatic cancer, and reduce the risk of Barrett's esophageal progression to esophageal adenocarcinoma. The protective effect of aspirin on CRC and colorectal adenomas is almost certain, especially in older adults at risk for CVD. In April 2016, the United States Preventive Services Task Force formally issued guidelines recommending LDA (75-100 mg/day) to prevent CVD and CRC in people aged 50–69 years with CVD risk but no elevated hemorrhage risk (11). However, the preventive effect for other GI cancer types is still controversial. The effect of aspirin on cancer prevention is mainly reflected after 4–5 years, even at low doses. However, a recent study (ASPREE trial) found that aspirin did not help reduce all-cause mortality (mainly cancer-related mortality, including GI cancer) (100). There are several possible reasons why the results of this study differ from those of previous studies: i) Most previous studies did not regard cancer as a predefined secondary endpoint, nor did they establish systematic approaches for the diagnosis and adjudication of cancer endpoints; and ii) the participants in the aforementioned study were mainly healthy individuals aged ≥70 years, with a median follow-up of 4.7 years, while previous studies mainly focused on people aged <70 and found that the effects of aspirin take 4–5 years to show. The other primary prevention trials of aspirin did not find similar results, suggesting that the deaths reported in the aforementioned study should be interpreted with caution.
Speculation remains as to the risk-benefit ratio of long-term aspirin use and the optimal dose required for effective chemoprevention while minimizing side effects. One of the most representative studies of the effects of different doses of aspirin on the risk of GI cancer is that conducted by Rothwell et al (35). The study found the same effect for 75–300 mg doses of aspirin with regard to the reduction of fatal CRC, with 75 mg daily being as effective as higher doses. The group also observed an absolute risk reduction in colorectal malignancy of ~1.5% after a 5-year period of treatment with at least 75 mg aspirin. However, very low doses of aspirin (e.g., 30 mg/day) are associated with a higher risk of fatal CRC compared with higher doses (34,35). The effective dose of aspirin in order to obtain the associated benefits of the drug in the GI tract varies between trials, although some studies have demonstrated that high-dose aspirin (>500 mg/day) may confer stronger protection than standard dose regimens (28,101). As summarized in Table I, numerous recent trials have challenged this notion, providing evidence for the use of LDA in cancer prevention. This phenomenon may be related to the different pharmacokinetics and pharmacodynamics of aspirin in different environments, and the physiological differences among patient populations (102,103).
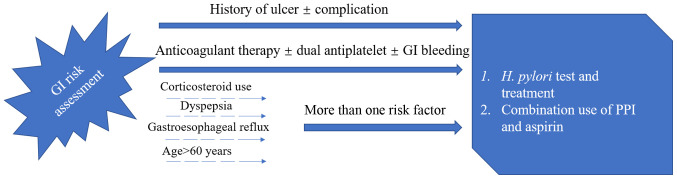
The main harmful effects of aspirin on the digestive system are bleeding, ulcers, perforation, dyspepsia and discomfort, while hepatic dysfunction is rare (Table II). One study found that aspirin has low toxicity and leads to a lower incidence of bleeding in the upper GI tract, and that it mainly results in dyspepsia. This study demonstrated that the beneficial effects of aspirin on cancer outweigh the risk of aspirin-induced bleeding events (104). The main risk factors for bleeding in aspirin users are age and H. pylori infection (7,105). Age is a key factor when weighing the pros and cons of aspirin. Once an individual approaches 60 years old, there is a marked increase in the risk of aspirin-induced bleeding events, with a non-linear relationship between increased risk and subsequent age (106,107). Changing drug formulations would not alleviate the adverse effects of aspirin on upper GI mucosa, and neither buffer nor enteric-coated tablets can reduce the risk of massive upper GI bleeding (107,108). According to previous studies, long-term LDA use for >4–5 years still plays a role in the prevention of GI cancer in healthy people aged 55–65 years. Since aspirin takes 4–5 years to have an effect and there is an increased risk of GI side effects in people aged >60, the prophylactic use of aspirin alone in people aged >60 is not recommended. Numerous studies have demonstrated that proton pump inhibitors (PPI) significantly reduce the risk of GI adverse events in aspirin patients (14,89,109–111). H2-receptor antagonists can also inhibit the secretion of gastric acid to a certain extent, but their effect may not be as good as that of PPI preparation (112). This superiority was observed uniformly in the separate clinical circumstances in which these agents are used in peptic ulcer disease, namely the prevention, healing and treatment of acute upper GI bleeding (113). According to the report by the American College of Cardiology Foundation Task Force (Fig. 1) (107), PPI were recommended in association with low-dose aspirin for patients with prior history of ulcer diseases or complicated GI ulcer. For other patients who have more than one risk factor among the categories of corticosteroid use, dyspepsia or gastroesophageal reflux and age >60 years, PPI are recommended. As H. pylori is an important risk factor for ulcer and ulcer bleeding in patients on LDA, a systematic testing for and eradication of H. pylori is recommended before starting aspirin for patients with prior history of ulcer disease (107). The value of PPI combined with aspirin lies not only in its ability to decrease the incidence of adverse events in the upper GI, but also in its ability to decrease the risk of withdrawal of aspirin (114,115). This has been confirmed in both RCTs and observational studies (114,115).
Figure 1.
According to the report of the American College of Cardiology Foundation Task Force (107), PPI recommendation for various low-dose aspirin users. PPI, proton pump inhibitor; H. pylori, helicobacter pylori; GI, gastrointestinal.
Clinicians always face clinical dilemmas and need to balance the risks and benefits of treatment. The use of aspirin in preventing GI cancer (or adenoma) and the risk of GI damage is one of these situations. In conclusion, the continued prophylactic use of LDA (75–325 mg/day) for at least 4–5 years to prevent GI cancer in non-high-risk populations aged 55–65 years should be considered for several reasons: i) A large amount of evidence suggests that LDA may play a prophylactic role in GI cancer. In addition, LDA causes less damage to the GI tract compared with high-dose aspirin. ii) The negative effects of aspirin to the GI tract are mainly the damage to the upper GI tract. The efficacy of PPI in treating LDA-related gastroduodenal ulcers and bleeding in most populations has been well documented and recognized. iii) Although, there is no effective preventive measure for lower GI bleeding, the clinical significance of LDA-induced lower GI mucosal injury is still unclear. Most studies investigating lower GI bleeding events had small sample sizes with wide CIs. LDA is routinely used in the primary and secondary prophylaxis of CVD. With a globally aging population (116), the consumption of LDA seems likely to increase in the future. Effects of aspirin need to be seen from a risk-benefit perspective, and from all the aforementioned evidence, we believe that the benefits outweigh the risks. However, unanswered questions related to the exact mechanism of cancer-related effects, optimal dose, duration, treatment options, and the balance of risks and benefits among specific populations need to be further investigated. The present review will contribute to continued progress in this exciting area of research.
Acknowledgments
Not applicable.
Glossary
Abbreviations
- GI
gastrointestinal
- LDA
low-dose aspirin
- NSAIDs
non-steroidal anti-inflammatory drugs
- FAP
familial adenomatous polyposis
- RCT
randomized controlled trials
- CRC
colorectal cancer
- CVD
cardiovascular disease
- NF-κB
nuclear factor κB
- PPI
proton pump inhibitor
- HCC
hepatocellular carcinoma
- ICC
intrahepatic cholangiocarcinoma
- COX-2
cyclooxygenase 2
- AMPK
adenosine monophosphate-activated protein kinase
- UGSs
upper GI symptoms
- H. pylori
Helicobacter pylori
- CI
confidence interval
Funding
This study was supported by grants from the Natural Science Foundation of Ningbo (grant no. 2016A610158), the Natural Science Foundation of Ningbo (grant no. 2014A610226) and the Scientific Benefit for People Project of Ningbo (grant no. 2014C51001).
Availability of data and materials
Not applicable.
Authors' contributions
ZL wrote the paper and collected the data. BS and ZW conceived and designed the review. CC and HS collected important background information, prepared the preliminary work of the manuscript and assisted in preliminary data collection. XD reviewed and edited the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Onyoh EF, Hsu WF, Chang LC, Lee YC, Wu MS, Chiu HM. The rise of colorectal cancer in asia: Epidemiology, screening, and management. Curr Gastroenterol Rep. 2019;21:36. doi: 10.1007/s11894-019-0703-8. [DOI] [PubMed] [Google Scholar]
- 4.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483–4490. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wick JY. Aspirin: A history, A love story. Consult Pharm. 2012;27:322–329. doi: 10.4140/TCP.n.2012.322. [DOI] [PubMed] [Google Scholar]
- 6.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC Guideline for the management of patients with Non-ST-Elevation acute coronary syndromes: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Colle Cardiol. 2014;64:2645–2687. doi: 10.1016/j.jacc.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Antithrombotic Trialists' (ATT) Collaboration, corp-author. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roffi M, Patrono C, Collet J, Mueller C, Valgimigli M, Andreotti F, Bax J, Borger M, Brotons C, Chew DP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-Segment Elevation of the European Society of Cardiology (ESC) G Ital Cardiol (Rome) 2016;17:831–872. doi: 10.1714/2464.25804. (In Italian) [DOI] [PubMed] [Google Scholar]
- 9.Smith DK, Demetriou T, Weber C. Aspirin for primary prevention: USPSTF recommendations for CVD and colorectal cancer. J Fam Pract. 2019;68:146–151. [PubMed] [Google Scholar]
- 10.Ventura L, Miccinesi G, Barchielli A, Manneschi G, Puliti D, Mantellini P, Orso F, Zappa M. Does low-dose aspirin use for cardiovascular disease prevention reduce colorectal cancer deaths? A comparison of two cohorts in the Florence district, Italy. Eur J Cancer Prev. 2018;27:134–139. doi: 10.1097/CEJ.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 11.Bibbins-Domingo K, U.S. Preventive Services Task Force Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:836–845. doi: 10.7326/M16-0577. [DOI] [PubMed] [Google Scholar]
- 12.Cea Soriano L, Soriano-Gabarró M, García Rodríguez LA. Incidence of colorectal cancer in new users and non-users of low-dose aspirin without existing cardiovascular disease: A cohort study using The Health Improvement Network. Int J Cardiol. 2017;248:376–381. doi: 10.1016/j.ijcard.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 13.Soon S, Chia WJ, Redekop K, Wee HL. A Cost-effectiveness analysis of aspirin in the primary prevention of cardiovascular diseases and colorectal cancer. Value Health. 2015;18:A462. doi: 10.1016/j.jval.2015.09.1200. [DOI] [Google Scholar]
- 14.Lanas A, Gargallo CJ. Management of low-dose aspirin and clopidogrel in clinical practice: A gastrointestinal perspective. J Gastroenterol. 2015;50:626–637. doi: 10.1007/s00535-015-1038-3. [DOI] [PubMed] [Google Scholar]
- 15.Lanas A, Scheiman J. Low-dose aspirin and upper gastrointestinal damage: Epidemiology, prevention and treatment. Curr Med Res Opin. 2007;23:163–173. doi: 10.1185/030079907X162656. [DOI] [PubMed] [Google Scholar]
- 16.Yuhara H, Corley DA, Nakahara F, Nakajima T, Koike J, Igarashi M, Suauki T, Mine T. Aspirin and non-aspirin NSAIDs increase risk of colonic diverticular bleeding: A systematic review and meta-analysis. J Gastroenterol. 2014;49:992–1000. doi: 10.1007/s00535-013-0905-z. [DOI] [PubMed] [Google Scholar]
- 17.Strum WB. Colorectal adenomas. N Engl J Med. 2016;374:1065–1075. doi: 10.1056/NEJMra1513581. [DOI] [PubMed] [Google Scholar]
- 18.Giovannucci E, Rimm E, Stampfer M, Colditz G, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994;121:241–246. doi: 10.7326/0003-4819-121-4-199408150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Thun MJ, Namboodiri MM, Heath C., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 20.Sandler R, Halabi S, Baron J, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas J, Karp D, Loprinzi CL, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 21.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 22.Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009;4:22. doi: 10.1186/1750-1172-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aihara H, Kumar N, Thompson CC. Diagnosis, surveillance, and treatment strategies for familial adenomatous polyposis: Rationale and update. Eur J Gastroenterol Hepatol. 2014;26:255–262. doi: 10.1097/MEG.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwama T, Tamura K, Morita T, Hirai T, Hasegawa H, Koizumi K, Shirouzu K, Sugihara K, Yamamura T, Muto T, et al. A clinical overview of familial adenomatous polyposis derived from the database of the polyposis registry of Japan. Int J Clin Oncol. 2004;9:308–316. doi: 10.1007/s10147-004-0414-4. [DOI] [PubMed] [Google Scholar]
- 25.Jasperson K, Burt RW. The genetics of colorectal cancer. Surg Oncol Clin N Am. 2015;24:683–703. doi: 10.1016/j.soc.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa H. Chemoprevention of carcinogenesis in familial tumors. Int J Clin Oncol. 2004;9:299–303. doi: 10.1007/s10147-004-0417-1. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa H, Wakabayashi K, Suzuki S, Mutoh M, Hirata K, Nakamura T, Takeyama I, Kawano A, Gondo N, Abe T, et al. Preventive effects of low-dose aspirin on colorectal adenoma growth in patients with familial adenomatous polyposis: Double-blind, randomized clinical trial. Cancer Med. 2013;2:50–56. doi: 10.1002/cam4.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burn J, Bishop DT, Chapman PD, Elliott F, Bertario L, Dunlop MG, Eccles D, Ellis A, Evans DG, Fodde R, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res (Phila) 2011;4:655–665. doi: 10.1158/1940-6207.CAPR-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cea Soriano L, Soriano-Gabarró M, Garcia Rodríiguez LA. Incidence of colorectal cancer in new users and non-users of low-dose aspirin without existing cardiovascular disease: A cohort study using The Health Improvement Network. Int J Cardiol. 2017;248:376–381. doi: 10.1016/j.ijcard.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y, Nishihara R, Wu K, Wang M, Ogino S, Willett WC, Spiegelman D, Fuchs CS, Giovannucci EL, Chan AT. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016;2:762–769. doi: 10.1001/jamaoncol.2015.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thun MJ, Henley SJ, Patrono C. Nonsteroidal Anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic, and clinical issues. J Nat Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 32.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: Analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 33.Chubak J, Whitlock EP, Williams SB, Kamineni A, Burda BU, Buist DS, Anderson ML. Aspirin for the prevention of cancer incidence and mortality: Systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:814–825. doi: 10.7326/M15-2117. [DOI] [PubMed] [Google Scholar]
- 34.Flossmann E, Rothwell PM, British Doctors Aspirin Trial and the UK-TIA Aspirin Trial Effect of aspirin on long-term risk of colorectal cancer: Consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 35.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 36.Li XF, Xu BZ, Wang SZ. Aspirin inhibits the proliferation and migration of gastric cancer cells in p53-knockout mice. Oncol Lett. 2016;12:3183–3186. doi: 10.3892/ol.2016.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ratnasinghe LD, Graubard BI, Kahle L, Tangrea JA, Taylor PR, Hawk E. Aspirin use and mortality from cancer in a prospective cohort study. Anticancer Res. 2004;24:3177–3184. [PubMed] [Google Scholar]
- 38.Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW., Jr Aspirin use and risk of fatal cancer. Cancer Res. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 39.Rothwell PM, Fowkes FGR, Belch JFF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 40.Farrow DC, Vaughan TL, Hansten PD, Stanford JL, Risch HA, Gammon MD, Chow WH, Dubrow R, Ahsan H, Mayne ST, et al. Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:97–102. [PubMed] [Google Scholar]
- 41.Vaughan TL, Dong LM, Blount PL, Ayub K, Odze RD, Sanchez CA, Rabinovitch PS, Reid BJ. Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett's oesophagus: A prospective study. Lancet Oncol. 2005;6:945–952. doi: 10.1016/S1470-2045(05)70431-9. [DOI] [PubMed] [Google Scholar]
- 42.González-Pérez A, Rodríguez LAG, LópezRidaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: A meta-analysis. BMC Cancer. 2003;3:28. doi: 10.1186/1471-2407-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu JF, Jamieson GG, Wu TC, Zhu GJ, Drew PA. A preliminary study on the postoperative survival of patients given aspirin after resection for squamous cell carcinoma of the esophagus or adenocarcinoma of the cardia. Ann Surg Oncol. 2009;16:1397–1402. doi: 10.1245/s10434-009-0382-z. [DOI] [PubMed] [Google Scholar]
- 44.Frouws MA, Bastiaannet E, Langley RE, Chia WK, van Herk-Sukel MP, Lemmens VE, Putter H, Hartgrink HH, Bonsing BA, Van de Velde CJ, et al. Effect of low-dose aspirin use on survival of patients with gastrointestinal malignancies; an observational study. Br J Cancer. 2017;116:405–413. doi: 10.1038/bjc.2016.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macfarlane TV, Murchie P, Watson MC. Aspirin and other non-steroidal anti-inflammatory drug prescriptions and survival after the diagnosis of head and neck and oesophageal cancer. Cancer Epidemiol. 2015;39:1015–1022. doi: 10.1016/j.canep.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 46.Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: A quantitative review to 2011. Ann Oncol. 2012;23:1403–1415. doi: 10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 47.Jankowski JAZ, de Caestecker J, Love SB, Reilly G, Watson P, Sanders S, Ang Y, Morris D, Bhandari P, Brooks C, et al. Esomeprazole and aspirin in Barrett's oesophagus (AspECT): A randomised factorial trial. Lancet. 2018;392:400–408. doi: 10.1016/S0140-6736(18)31388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spence AD, Busby J, Johnston BT, Baron JA, Hughes CM, Coleman HG, Cardwell CR. Low-Dose aspirin use does not increase survival in 2 independent population-based cohorts of patients with esophageal or gastric cancer. Gastroenterology. 2018;154:849–860.e1. doi: 10.1053/j.gastro.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 49.Choi JH, Lee SH, Huh G, Chun JW, You MS, Paik WH, Ryu JK, Kim YT. The association between use of statin or aspirin and pancreatic ductal adenocarcinoma: A nested case-control study in a Korean nationwide cohort. Cancer Med. 2019;8:7419–7430. doi: 10.1002/cam4.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahasrabuddhe VV, Gunja MZ, Graubard BI, Trabert B, Schwartz LM, Park Y, Hollenbeck AR, Freedman ND, McGlynn KA. Nonsteroidal Anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104:1808–1814. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coogan PF, Rosenberg L, Palmer JR, Strom BL, Zauber AG, Stolley PD, Shapiro S. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol Biomarkers Prev. 2000;9:119–123. [PubMed] [Google Scholar]
- 52.Petrick JL, Sahasrabuddhe VV, Chan AT, Alavanja MC, Beane-Freeman LE, Buring JE, Chen J, Chong DQ, Freedman ND, Fuchs CS, et al. NSAID use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The liver cancer pooling project. Cancer Prev Res. 2015;8:1156–1162. doi: 10.1158/1940-6207.CAPR-15-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2013;11:45–54. doi: 10.1038/nrgastro.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hawk ET, Umar A, Viner JL. Colorectal cancer chemoprevention-an overview of the science 1 1 This article was prepared in our capacity as employees of the U.S. Federal Government. Gastroenterology. 2004;126:1423–1447. doi: 10.1053/j.gastro.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–1309. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 56.Pasche B, Wang M, Pennison M, Jimenez H. Prevention and treatment of cancer with aspirin: Where do we stand? Semin Oncol. 2014;41:397–401. doi: 10.1053/j.seminoncol.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahboubi Rabbani SMI, Zarghi A. Selective COX-2 inhibitors as anticancer agents: A patent review (2014–2018) Expert Opin Ther Pat. 2019;29:407–427. doi: 10.1080/13543776.2019.1623880. [DOI] [PubMed] [Google Scholar]
- 58.Poorani R, Bhatt AN, Dwarakanath BS, Das UN. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur J Pharmacol. 2016;785:116–132. doi: 10.1016/j.ejphar.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 59.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chell S, Kaidi A, Williams AC, Paraskeva C. Mediators of PGE2 synthesis and signalling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer. Biochim Biophys Acta. 2006;1766:104–119. doi: 10.1016/j.bbcan.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Z, Dubois RN. Par-4, a proapoptotic gene, is regulated by NSAIDs in human colon carcinoma cells. Gastroenterol. 2000;118:1012–1017. doi: 10.1016/S0016-5085(00)84303-6. [DOI] [PubMed] [Google Scholar]
- 64.Kashfi K, Rigas B. Non-COX-2 targets and cancer: Expanding the molecular target repertoire of chemoprevention. Biochem Pharmacol. 2005;70:969–986. doi: 10.1016/j.bcp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Shureiqi I, Xu X, Chen D, Lotan R, Morris JS, Fischer SM, Lippman SM. Nonsteroidal anti-inflammatory drugs induce apoptosis in esophageal cancer cells by restoring 15-lipoxygenase-1 expression. Cancer Res. 2001;61:4879–4884. [PubMed] [Google Scholar]
- 66.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McIlhatton MA, Tyler J, Burkholder S, Ruschoff J, Rigas B, Kopelovich L, Fishel R. Nitric Oxide-donating aspirin derivatives suppress microsatellite instability in mismatch repair-deficient and hereditary nonpolyposis colorectal cancer cells. Cancer Res. 2007;67:10966–10975. doi: 10.1158/0008-5472.CAN-07-2562. [DOI] [PubMed] [Google Scholar]
- 68.Rüschoff J, Wallinger S, Dietmaier W, Bocker T, Brockhoff G, Hofstädter F, Fishel R. Aspirin suppresses the mutator phenotype associated with hereditary nonpolyposis colorectal cancer by genetic selection. Proc Natl Acad Sci USA. 1998;95:11301–11306. doi: 10.1073/pnas.95.19.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goel A, Chang DK, Ricciardiello L, Gasche C, Boland CR. A novel mechanism for aspirin-mediated growth inhibition of human colon cancer cells. Clin Cancer Res. 2003;9:383–390. [PubMed] [Google Scholar]
- 70.Huang Z, Fang W, Liu W, Wang L, Liu B, Liu S, Liu S. Aspirin induces Beclin-1-dependent autophagy of human hepatocellular carcinoma cell. Eur J Pharmacol. 2018;823:58–64. doi: 10.1016/j.ejphar.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 71.Bhattacharya A, Eissa NT. Autophagy and autoimmunity crosstalks. Front Immunol. 2013;4:88. doi: 10.3389/fimmu.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henry WS, Laszewski T, Tsang T, Beca F, Beck AH, McAllister SS, Toker A. Aspirin suppresses growth in PI3K-mutant breast cancer by activating AMPK and inhibiting mTORC1 signaling. Cancer Res. 2017;77:790–801. doi: 10.1158/0008-5472.CAN-16-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu W, Jiang Y, Sun J, Geng S, Pan Z, Prinz RA, Wang C, Sun J, Jiao X, Xu X. Activation of TGF-β-activated kinase 1 (TAK1) restricts Salmonella Typhimurium growth by inducing AMPK activation and autophagy. Cell Death Dis. 2018;9:570. doi: 10.1038/s41419-018-0612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hardie DG, Ross FA, Hawley SA. AMP-Activated protein kinase: A target for drugs both ancient and modern. Chem Biol. 2012;19:1222–1236. doi: 10.1016/j.chembiol.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kostadinov RL, Kuhner MK, Li X, Sanchez CA, Galipeau PC, Paulson TG, Sather CL, Srivastava A, Odze RD, Blount PL, et al. NSAIDs modulate clonal evolution in Barrett's esophagus. PLoS Genet. 2013;9:e1003553. doi: 10.1371/journal.pgen.1003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: The promise of precision chemoprevention. Nat Rev Cancer. 2016;16:173–186. doi: 10.1038/nrc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gala MK, Chan AT. Molecular pathways: Aspirin and Wnt signaling-a molecularly targeted approach to cancer prevention and treatment. Clin Cancer Res. 2015;21:1543–1548. doi: 10.1158/1078-0432.CCR-14-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeomans ND, Lanas AI, Talley NJ, Thomson AB, Daneshjoo R, Eriksson B, Appelman-Eszczuk S, Långström G, Naesdal J, Serrano P, et al. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharmacol Ther. 2005;22:795–801. doi: 10.1111/j.1365-2036.2005.02649.x. [DOI] [PubMed] [Google Scholar]
- 79.Cayla G, Collet JP, Silvain J, Thiefin G, Woimant F, Montalescot G. Prevalence and clinical impact of Upper Gastrointestinal Symptoms in subjects treated with low dose aspirin: The UGLA survey. Int J Cardiol. 2012;156:69–75. doi: 10.1016/j.ijcard.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 80.Biondi-Zoccai GG, Lotrionte M, Agostoni P, Abbate A, Fusaro M, Burzotta F, Testa L, Sheiban I, Sangiorgi G. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J. 2006;27:2667–2674. doi: 10.1093/eurheartj/ehl334. [DOI] [PubMed] [Google Scholar]
- 81.Baron JA, Senn S, Voelker M, Lanas A, Laurora I, Thielemann W, Bruckner A, McCarthy D. Gastrointestinal adverse effects of short-term aspirin use: A meta-analysis of published randomized controlled trials. Drugs R D. 2013;13:9–16. doi: 10.1007/s40268-013-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cayla G, Collet JP, Silvain J, Thiefin G, Woimant F, Montalescot G. Prevalence and clinical impact of Upper Gastrointestinal Symptoms in subjects treated with low dose aspirin: The UGLA survey. Int J Cardiol. 2012;156:69–75. doi: 10.1016/j.ijcard.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 83.Yeomans ND, Naesdal J. Systematic review: Ulcer definition in NSAID ulcer prevention trials. Aliment Pharmacol Ther. 2008;27:465–472. doi: 10.1111/j.1365-2036.2008.03610.x. [DOI] [PubMed] [Google Scholar]
- 84.Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology. 1999;117:17–25. doi: 10.1016/S0016-5085(99)70545-7. [DOI] [PubMed] [Google Scholar]
- 85.Papatheodoridis GV, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: Systematic review. Clin Gastroenterol Hepatol. 2006;4:130–142. doi: 10.1016/j.cgh.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 86.Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: A meta-analysis. Lancet. 2002;359:14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 87.Thorat MA, Cuzick J. Prophylactic use of aspirin: Systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5–18. doi: 10.1007/s10654-014-9971-7. [DOI] [PubMed] [Google Scholar]
- 88.Iwamoto J, Mizokami Y, Shimokobe K, Ito M, Hirayama T, Saito Y, Ikegami T, Honda A, Matsuzaki Y. Clinical features of gastroduodenal ulcer in Japanese patients taking low-dose aspirin. Dig Dis Sci. 2010;55:2270–2274. doi: 10.1007/s10620-009-1009-8. [DOI] [PubMed] [Google Scholar]
- 89.Garcia Rodriguez LA, Martin-Perez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding Risk with long-term low-dose aspirin: A systematic review of observational studies. PLoS One. 2016;11:e0160046. doi: 10.1371/journal.pone.0160046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, Reid CM, Lockery JE, Kirpach B, Storey E, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–1518. doi: 10.1056/NEJMoa1805819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Abajo FJ, García Rodríguez LA. Risk of upper gastrointestinal bleeding and perforation associated with low-dose aspirin as plain and enteric-coated formulations. BMC Clin Pharmacol. 2001;1:1. doi: 10.1186/1472-6904-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lanas A, Serrano P, Bajador E, Esteva F, Benito R, Sáinz R. Evidence of aspirin use in both upper and lower gastrointestinal perforation. Gastroenterology. 1997;112:683–689. doi: 10.1053/gast.1997.v112.pm9041228. [DOI] [PubMed] [Google Scholar]
- 93.Yamada A, Sugimoto T, Kondo S, Ohta M, Watabe H, Maeda S, Togo G, Yamaji Y, Ogura K, Okamoto M, et al. Assessment of the risk factors for colonic diverticular hemorrhage. Dis Colon Rectum. 2008;51:116–120. doi: 10.1007/s10350-007-9137-8. [DOI] [PubMed] [Google Scholar]
- 94.Lanas Á, Carrera-Lasfuentes P, Arguedas Y, García S, Bujanda L, Calvet X, Ponce J, Perez-Aísa Á, Castro M, Muñoz M, et al. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin Gastroenterol Hepatol. 2015;13:906–912.e2. doi: 10.1016/j.cgh.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 95.Hirata Y, Kataoka H, Shimura T, Mizushima T, Mizoshita T, Tanida S, Kamiya T, Joh T. Incidence of gastrointestinal bleeding in patients with cardiovascular disease: Buffered aspirin versus enteric-coated aspirin. Scand J Gastroenterol. 2011;46:803–809. doi: 10.3109/00365521.2011.568522. [DOI] [PubMed] [Google Scholar]
- 96.Nagata N, Niikura R, Aoki T, Shimbo T, Kishida Y, Sekine K, Tanaka S, Watanabe K, Sakurai T, Yokoi C, et al. Colonic diverticular hemorrhage associated with the use of nonsteroidal anti-inflammatory drugs, low-dose aspirin, antiplatelet drugs, and dual therapy. J Gastroenterol Hepatol. 2014;29:1786–1793. doi: 10.1111/jgh.12595. [DOI] [PubMed] [Google Scholar]
- 97.Reichert MC, Krawczyk M, Appenrodt B, Casper M, Friesenhahn-Ochs B, Grünhage F, Jüngst C, Zimmer V, Lammert F, Dauer M. Selective association of nonaspirin NSAIDs with risk of diverticulitis. Int J Colorectal Dis. 2018;33:423–430. doi: 10.1007/s00384-018-2968-z. [DOI] [PubMed] [Google Scholar]
- 98.Strate LL, Liu YL, Huang ES, Giovannucci EL, Chan AT. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology. 2011;140:1427–1433. doi: 10.1053/j.gastro.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O'Connor N, Dargan PI, Jones AL. Hepatocellular damage from non-steroidal anti-inflammatory drugs. QJM. 2003;96:787–791. doi: 10.1093/qjmed/hcg138. [DOI] [PubMed] [Google Scholar]
- 100.McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, Kirpach B, Shah RC, Ives DG, Storey E, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519–1528. doi: 10.1056/NEJMoa1803955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: An analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Würtz M, Grove EL. Interindividual variability in the efficacy of oral antiplatelet drugs: Definitions, mechanisms and clinical importance. Curr Pharm Des. 2012;18:5344–5361. doi: 10.2174/138161212803251925. [DOI] [PubMed] [Google Scholar]
- 103.Rocca B, Petrucci G. Variability in the responsiveness to low-dose aspirin: Pharmacological and disease-related mechanisms. Thrombosis. 2012;2012:376721. doi: 10.1155/2012/376721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Joharatnam-Hogan N, Cafferty F, Hubner R, Swinson D, Sothi S, Gupta K, Falk S, Patel K, Warner N, Kunene V, et al. Aspirin as an adjuvant treatment for cancer: Feasibility results from the Add-Aspirin randomised trial. Lancet Gastroenterol Hepatol. 2019;4:854–862. doi: 10.1016/S2468-1253(19)30289-4. [DOI] [PubMed] [Google Scholar]
- 105.Kaufman DW, Kelly JP, Wiholm BE, Laszlo A, Sheehan JE, Koff RS, Shapiro S. The risk of acute major upper gastrointestinal bleeding among users of aspirin and ibuprofen at various levels of alcohol consumption. Am J Gastroenterol. 1999;94:3189–3196. doi: 10.1111/j.1572-0241.1999.01517.x. [DOI] [PubMed] [Google Scholar]
- 106.Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22–20. doi: 10.1186/1741-7015-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD, Johnson DA, Mahaffey KW, Quigley EM, American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: A Report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008;118:1894–1909. doi: 10.1161/CIRCULATIONAHA.108.191087. [DOI] [PubMed] [Google Scholar]
- 108.Kelly JP, Kaufman DW, Jurgelon JM, Sheehan J, Koff RS, Shapiro S. Risk of aspirin-associated major upper-gastrointestinal bleeding with enteric-coated or buffered product. Lancet. 1996;348:1413–1416. doi: 10.1016/S0140-6736(96)01254-8. [DOI] [PubMed] [Google Scholar]
- 109.Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71–79. doi: 10.1053/j.gastro.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 110.Sylvester KW, Cheng JW, Mehra MR. Esomeprazole and aspirin fixed combination for the prevention of cardiovascular events. Vasc Health Risk Manag. 2013;9:245–254. doi: 10.2147/VHRM.S44265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lanas A, Polo-Tomas M, Casado-Arroyo R. The aspirin cardiovascular/gastrointestinal risk calculator-a tool to aid clinicians in practice. Aliment Pharmacol Ther. 2013;37:738–748. doi: 10.1111/apt.12240. [DOI] [PubMed] [Google Scholar]
- 112.Mo C, Sun G, Lu ML, Zhang L, Wang YZ, Sun X, Yang YS. Proton pump inhibitors in prevention of low-dose aspirin-associated upper gastrointestinal injuries. World J Gastroenterol. 2015;21:5382–5392. doi: 10.3748/wjg.v21.i17.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scally B, Emberson JR, Spata E, Reith C, Davies K, Halls H, Holland L, Wilson K, Bhala N, Hawkey C, et al. Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: A meta-analysis of randomised trials. Lancet Gastroenterol Hepatol. 2018;3:231–241. doi: 10.1016/S2468-1253(18)30037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Whellan DJ, Goldstein JL, Cryer BL, Eisen GM, Lanas A, Miller AB, Scheiman JM, Fort JG, Zhang Y, O'Connor C. PA32540 (a coordinated-delivery tablet of enteric-coated aspirin 325 mg and immediate-release omeprazole 40 mg) versus enteric-coated aspirin 325 mg alone in subjects at risk for aspirin-associated gastric ulcers: Results of two 6-month, phase 3 studies. Am Heart J. 2014;168:495–502.e4. doi: 10.1016/j.ahj.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 115.Martín Merino E, Johansson S, Nagy P, García Rodríguez LA. Effect of baseline gastrointestinal risk and use of proton pump inhibitors on frequency of discontinuation of aspirin for secondary cardiovascular prevention in united kingdom primary care. Am J Cardiol. 2013;112:1075–1082. doi: 10.1016/j.amjcard.2013.05.051. [DOI] [PubMed] [Google Scholar]
- 116.Dey AB. World report on ageing and health. Indian J Med Res. 2017;145:150. doi: 10.4103/0971-5916.207249. [DOI] [Google Scholar]
- 117.Hull MA, Sprange K, Hepburn T, Tan W, Shafayat A, Rees CJ, Clifford G, Logan RF, Loadman PM, Williams EA, et al. Eicosapentaenoic acid and aspirin, alone and in combination, for the prevention of colorectal adenomas (seAFOod Polyp Prevention trial): A multicentre, randomised, double-blind, placebo-controlled, 2×2 factorial trial. Lancet. 2018;392:2583–2594. doi: 10.1016/S0140-6736(18)31775-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, Chaussade S, Baron JA. Aspirin for the chemoprevention of colorectal adenomas: Meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101:256–266. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: Long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159:77–85. doi: 10.7326/0003-4819-159-2-201307160-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kuan YC, Huang KW, Lin CL, Luo JC, Kao CH. Effects of aspirin or clopidogrel on colorectal cancer chemoprevention in patients with type 2 diabetes mellitus. Cancers (Basel) 2019;11:1468. doi: 10.3390/cancers11101468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 122.Chan FK, Leung Ki EL, Wong GL, Ching JY, Tse YK, Au KW, Wu JC, Ng SC. Risks of bleeding recurrence and cardiovascular events with continued aspirin use after lower gastrointestinal hemorrhage. Gastroenterology. 2016;151:271–277. doi: 10.1053/j.gastro.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 123.Casado Arroyo R, Polo-Tomas M, Roncales MP, Scheiman J, Lanas A. Lower GI bleeding is more common than upper among patients on dual antiplatelet therapy: Long-term follow-up of a cohort of patients commonly using PPI co-therapy. Heart. 2012;98:718–723. doi: 10.1136/heartjnl-2012-301632. [DOI] [PubMed] [Google Scholar]
- 124.Smecuol E, Pinto Sanchez MI, Suarez A, Argonz JE, Sugai E, Vazquez H, Litwin N, Piazuelo E, Meddings JB, Bai JC, Lanas A. Low-dose aspirin affects the small bowel mucosa: Results of a pilot study with a multidimensional assessment. Clin Gastroenterol Hepatol. 2009;7:524–529. doi: 10.1016/j.cgh.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 125.Moore RA, Derry S, McQuay HJ. Faecal blood loss with aspirin, nonsteroidal anti-inflammatory drugs and cyclo-oxygenase-2 selective inhibitors: Systematic review of randomized trials using autologous chromium-labelled erythrocytes. Arthritis Res Ther. 2008;10:R7. doi: 10.1186/ar2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Endo H, Higurashi T, Hosono K, Sakai E, Sekino Y, Iida H, Sakamoto Y, Koide T, Takahashi H, Yoneda M, et al. Efficacy of Lactobacillus casei treatment on small bowel injury in chronic low-dose aspirin users: A pilot randomized controlled study. J Gastroenterol. 2011;46:894–905. doi: 10.1007/s00535-011-0410-1. [DOI] [PubMed] [Google Scholar]
- 127.Montalto M, Gallo A, Curigliano V, D'Onofrio F, Santoro L, Covino M, Dalvai S, Gasbarrini A, Gasbarrini G. Clinical trial: The effects of a probiotic mixture on non-steroidal anti-inflammatory drug enteropathy-a randomized, double-blind, cross-over, placebo-controlled study. Aliment Pharmacol Ther. 2010;32:209–214. doi: 10.1111/j.1365-2036.2010.04324.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.