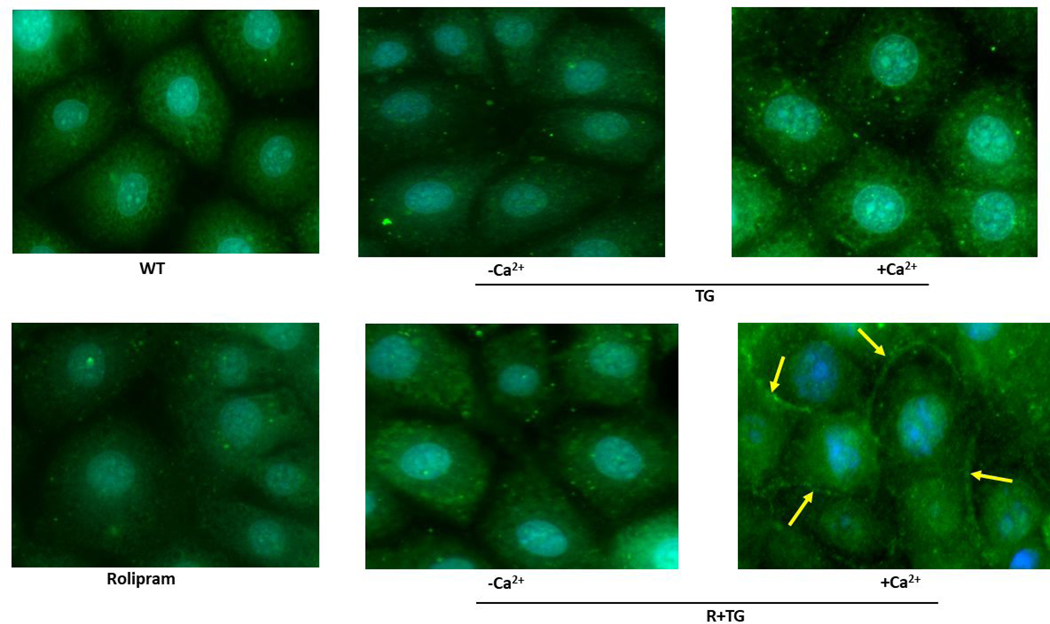
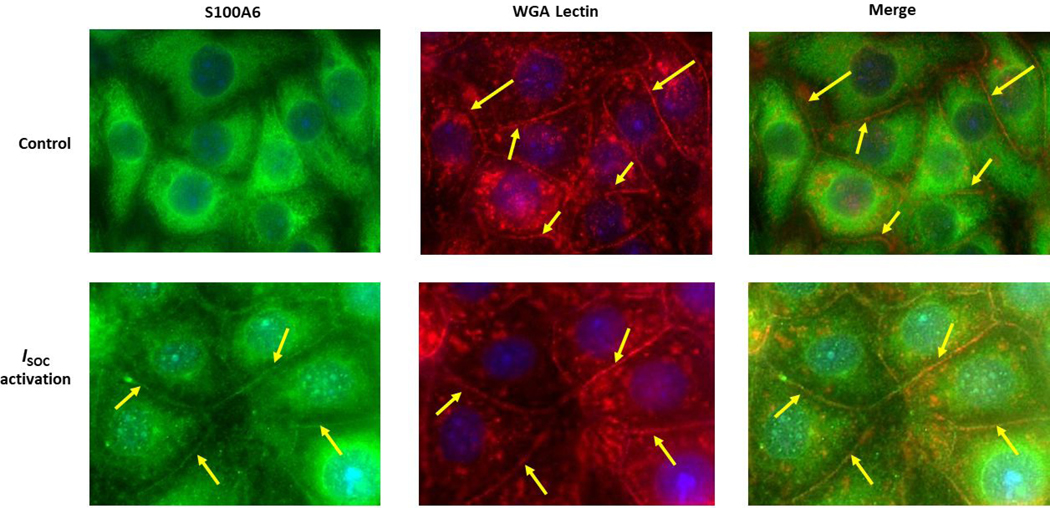
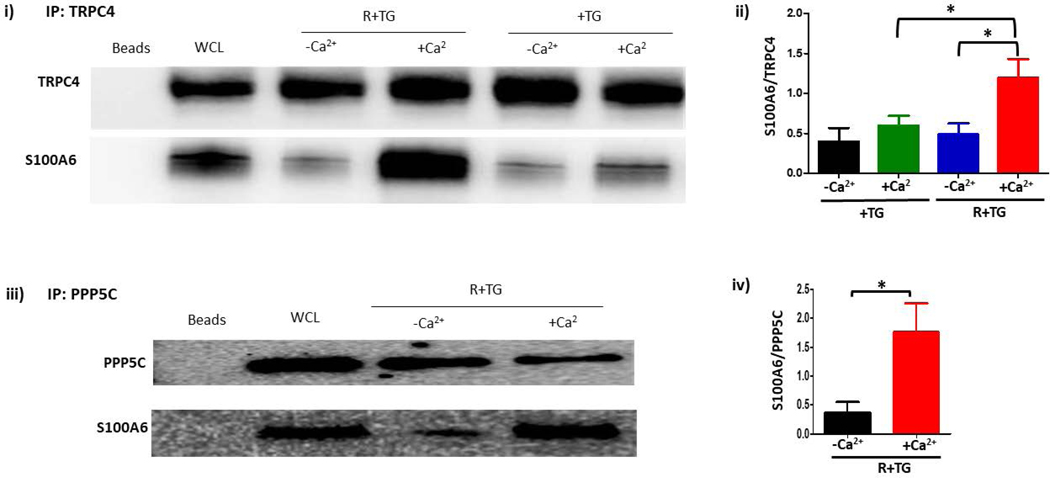
Figure 2. S100A6 translocates to the plasma membrane in a calcium-dependent manner to interact with the ISOC channel heterocomplex.
A.1. Immunocytochemistry for S100A6 was performed to determine cellular localization. In unstimulated PMVECs (WT), S100A6 is principally cytosolic. Treatment of PMVECs with thapsigargin alone (TG) in the absence of extracellular calcium or treatment with rolipram alone did not induce S100A6 translocation. In the presence of extracellular calcium, TG treatment caused only minor and infrequent S100A6 translocation. Upon activation of ISOC (R+TG) in the presence of extracellular calcium, part of the cytosolic S100A6 pool translocated to the plasma membrane particularly at the sites of cell-cell adhesion (yellow arrows). Images are representative from 3 independent experiments. A.2. Colocalization of S100A6 with TRITC-conjugated WGA lectin. In control PMVECs in which ISOC was not activated, no S100A6 was found at the membrane, which was labelled by WGA (red stain denoted by yellow arrows). In PMVECs in which ISOC was activated, S100A6 (green) colocalized with WGA (red) at the membrane (yellow arrows). Images are representative from 3 independent experiments. B. TRPC4 was immunoprecipitated from PMVECs following ISOC activation (R+TG) or following activation of other calcium channels (TG). Immunoprecipitations were performed in the presence or absence of extracellular calcium, and co-precipitation of S100A6 was probed. (i) In the absence of primary antibody (beads only), no bands were detected. Sample input of whole cell lysates (WCL) revealed presence of both TRPC4 and S100A6. While TRPC4 was immunoprecipitated following R+TG in both the presence and absence of extracellular calcium, co-precipitation of S100A6 was predominantly observed in the presence of extracellular calcium. Similarly, following activation of other calcium channels (TG), TRPC4 was immunoprecipitated in both the presence and absence of extracellular calcium. However, while some co-precipitation of S100A6 was observed in the presence of extracellular calcium, it was less than that observed following R+TG. (ii) Quantitation of S100A6 co-precipitation was assessed via densitometry of TRPC4 and S100A6 bands and the S100A6/TRPC4 ratio determined. Molecular weights observed: TRPC4 ~80 kDa; S100A6 10 kDa. n = 5 independent experiments. (iii) PPP5C was immunoprecipitated from PMVECs following ISOC activation (R+TG) in the presence or absence of extracellular calcium and co-precipitation of S100A6 probed. In the absence of primary antibody (beads only), no bands were detected, while WCL input revealed presence of both PPP5C and S100A6. Following ISOC activation (R+TG), robust co-precipitation of S100A6 was observed in the presence of extracellular calcium while only minor co-precipitation of S100A6 was observed in the absence of extracellular calcium. (iv) Quantitation of S100A6 co-precipitation was assessed via densitometry of PPP5C and S100A6 bands and the S100A6/PPP5C ratio determined. Molecular weights observed: PPP5C ~58 kDa; S100A6 10 kDa. n = 3 independent experiments.