Abstract
Chronic lung diseases (CLDs), such as chronic obstructive pulmonary disease, interstitial lung disease, and lung cancer, are among the leading causes of morbidity globally and impose major health and financial burdens on patients and society. Effective treatments are scarce, and relevant human model systems to effectively study CLD pathomechanisms and thus discover and validate potential new targets and therapies are needed. Precision-cut lung slices (PCLS) from healthy and diseased human tissue represent one promising tool that can closely recapitulate the complexity of the lung’s native environment, and recently, improved methodologies and accessibility to human tissue have led to an increased use of PCLS in CLD research. Here, we discuss approaches that use human PCLS to advance our understanding of CLD development, as well as drug discovery and validation for CLDs. PCLS enable investigators to study complex interactions among different cell types and the extracellular matrix in the native three-dimensional architecture of the lung. PCLS further allow for high-resolution (live) imaging of cellular functions in several dimensions. Importantly, PCLS can be derived from diseased lung tissue upon lung surgery or transplantation, thus allowing the study of CLDs in living human tissue. Moreover, CLDs can be modeled in PCLS derived from normal lung tissue to mimic the onset and progression of CLDs, complementing studies in end-stage diseased tissue. Altogether, PCLS are emerging as a remarkable tool to further bridge the gap between target identification and translation into clinical studies, and thus open novel avenues for future precision medicine approaches.
Keywords: PCLS, ex vivo lung disease, drug discovery, translation
Chronic lung diseases (CLDs) are among the leading causes of death and impose major health and financial burdens on patients and society. The pathology of CLDs is complex and heterogeneous, and the discovery and development of new therapeutics remains a challenging task. Most lung diseases have no cure (1). CLDs, such as chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF), are progressive and fatal, and the complex etiology and cellular pathomechanisms of these diseases remain poorly understood (2, 3). Although several different genetic and environmental associations have been identified to contribute to the onset and progression of CLDs, therapeutic interventions remain limited, and existing treatments largely target symptomatic relief. Only very recently have the first therapeutics for IPF entered into clinical use (4, 5). However, although these therapies have been shown to slow down disease progression, they are unable to stop or reverse it (6). Thus, there is an urgent unmet need to identify more effective and targeted treatments. Novel approaches and clinically relevant model systems are needed to explore disease pathomechanisms and, more importantly, to validate potential new targets and drugs. Although animal models have contributed tremendously to our understanding of disease pathomechanisms and remain invaluable for advancing our understanding of in vivo mechanisms, they do not necessarily mimic the nature and complexity of native human tissue, and in some cases, such as with species-specific infections, animals cannot be used to model human disease (7–10). Additional and complementary models are needed, which also will enable reduction and refinement of animal research.
Precision-cut lung slices (PCLS) (also referred to as lung tissue slices or lung tissue cultures in the literature [further discussed below]) provide an emerging and exciting opportunity to advance CLD research and validate novel therapies. In this review, we will summarize the methodological development of PCLS over the past few decades, and in particular discuss the recent applications of PCLS for acute lung disease and CLD modeling. We will focus primarily on chronic parenchymal lung diseases (IPF, COPD, and lung cancer), in which several novel advances have been made in recent years. We refer the reader to excellent reviews of PCLS applications to study airway function and contractility with regard to asthma and toxicological studies (see References 11–13). We will discuss disease-specific approaches, benefits, limitations, and the translational potential for CLDs. Whenever possible, we will focus on human PCLS models, but when human models are lacking or underdeveloped, we will refer to studies in rodent PCLS.
PCLS Development: Overview
Precision-cut tissue slices can be generated from various tissues, such as liver, brain, and lung. PCLS, in particular, can be generated from various anatomical locations of the lung (distal and proximal) and different species, including rodents, pigs, monkeys, and humans. Surgical resections from healthy and diseased human lung tissue have been a major source for advancing the use of PCLS.
PCLS from human lung tissue were first described in 1994 by Fisher and colleagues (13), and in subsequent years, human PCLS were used mainly for short-term toxicological studies (14). The generation of lung slices has gradually improved, extending cell viability over longer culture times and thus enabling more in-depth investigations of cellular functions and phenotypes, in particular by incorporating high-resolution imaging studies (15–19) (Figure 1). Regarding PCLS generation, several technical aspects are important as each of them can affect the suitability for specific studies and disease models. For further detailed descriptions of the “hands-on” generation of mouse and human PCLS, we refer the reader to several recently published protocols, which can be helpful when the PCLS methodology is newly set up. For example, it has been shown that lung storage before agarose filling, the filling itself, which device is used for slicing, and how the PCLS are cultured can significantly impact tissue viability and structure (20–23). Moreover, to model the in vivo environment, several studies have cultured PCLS on stretching devices (24–28). Different anatomical regions (such as the parenchyma), as well as distinct cell types, are relevant in modeling CLDs, and disease-directed protocols for generation and culture need to be considered (summarized in Table 1).
Figure 1.
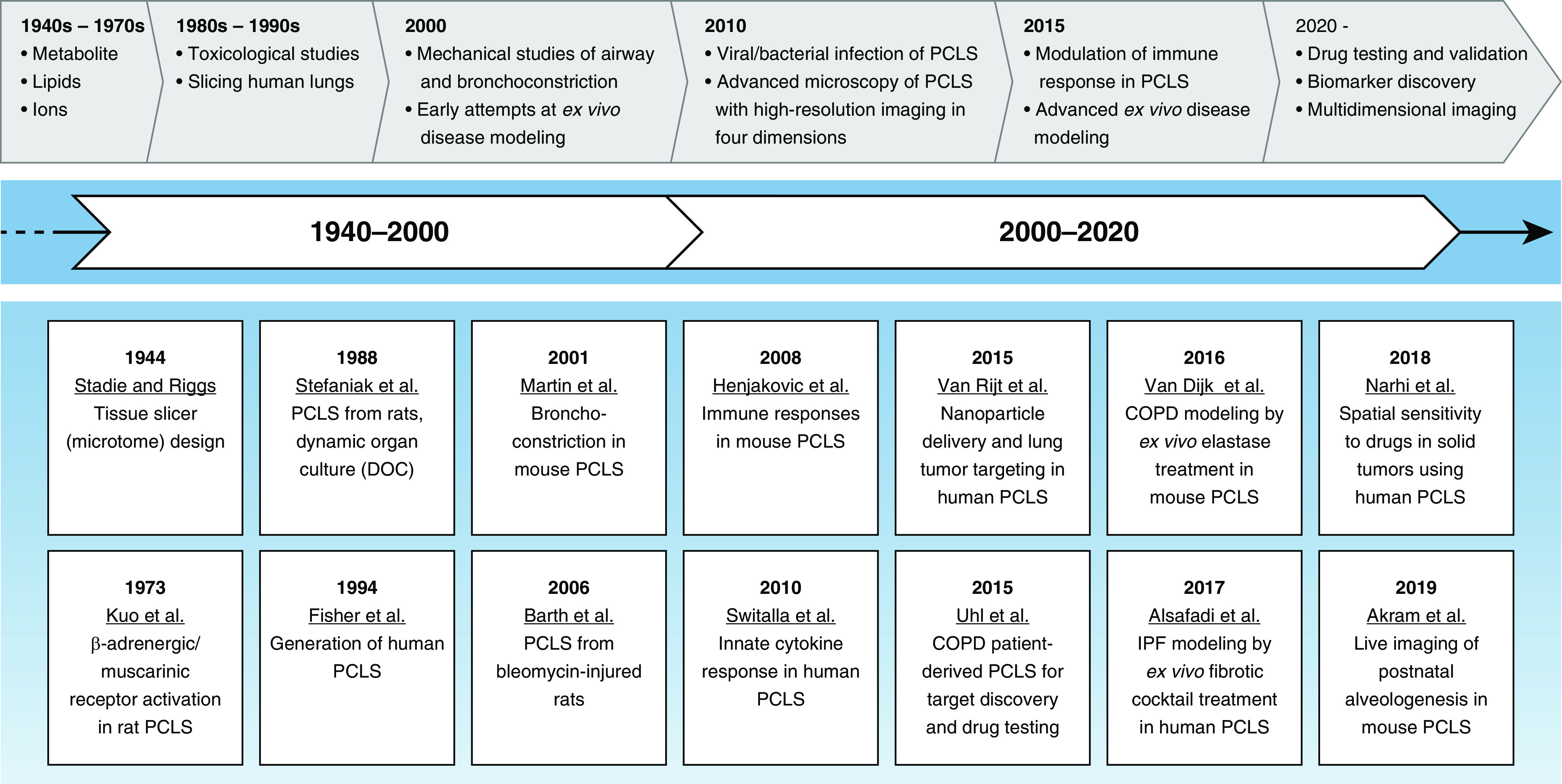
Development of precision-cut lung slices (PCLS) generation, leading to disease modeling and drug discovery. Ex vivo culturing of lung tissue has been performed since the early 1940s, although limited progress was made in the technique for the first two decades. Early studies were limited to short culture times and analyses of metabolites and toxicological studies. Mechanistic studies did not start until the 1970s. Precise and reproducible generation of thin lung slices began at the end of the 1980s, when agar was used to support the three-dimensional structure. The first lung slice from human lungs was generated in the early to mid 1990s. Most recently, lung slices from animal and human tissues have been used to explore in-depth mechanisms of lung diseases. COPD = chronic obstructive pulmonary disease; DOC = dynamic organ culture; IPF = idiopathic pulmonary fibrosis.
Table 1.
Technical Differences in the Generation of Precision-Cut Lung Slices
| Step | Property | Variation | Notes/Examples |
|---|---|---|---|
| Lung filling | Agarose (%) | • 0.4–1.5 (36, 57, 72) | • Lower percentages are used in rodents, and larger ones are used in human tissue and bigger animals |
| • 1.5–3 (15, 46) | |||
| • The percentage of agarose may affect the mechanical properties of the filled tissue | |||
| Media | • Salt solutions: PBS, EBSS, and HBSS (33, 73, 74) | • Some protocols add more supplements to the filling media, such as HEPES, penicillin/streptomycin, and Amphotericin B | |
| • Cell culture medium: (D)MEM, DMEM/F-12, RPMI) (48, 72) | • The choice of filling media may influence the quality and survival of specific cell types within the PCLS | ||
| Lung slicing | Media | • Salt-buffered solutions (16, 73, 75, 76) | • Most protocols use culture media for slicing with no alterations |
| • Cell culture medium (15, 48, 57, 77) | • Some protocols supplement media with CaCl2, MgSO4, KCl, NaCl, NaH2PO4, glucose, NaHCO3, HEPES, penicillin/streptomycin, insulin, hydrocortisone, retinyl acetate, gentamicin, or Amphotericin B | ||
| Devices | Tissue slicers (rotary chopping) (29) | • Krumdieck Slicer | |
| • Compresstome VF-300 | |||
| • Brendel Vitron Tissue Slicer | |||
| Vibratome (74) | • Leica Vibratome VT1000 | ||
| • Zeiss Hyrax Vibratory Microtome V55 | |||
| • Campden 7000 SMZ-2 | |||
| Thickness | • 150–225 μm (12, 74) | • Thinner PCLS are generally obtained from rodent tissue and allow for easier nutrient perfusion | |
| • 250–500 μm (24, 48, 72, 73, 78) | |||
| • Thicker PCLS are generally obtained from larger animal tissues or human tissue | |||
| • No perfusion/diffusion problems have been reported for PCLS 500 μm thickness or less | |||
| • Thicker PCLS allow for visualization of full alveolar spaces, improving the modeling aspects of specific diseases | |||
| • The thickness of PCLS can vary for different CLDs | |||
| • Slice thickness is usually optimized differently in each laboratory based on the research question | |||
| Culture | Preparation | Agarose removal (79) | • Some protocols attempt to remove agarose from PCLS by repetitive washes within the first few hours after slicing |
| • Agarose removal may allow for easier access to nutrients | |||
| Pretreatment | • Most protocols replace media with varying initial incubation periods | ||
| • Washing may be important for removal of secreted factors that may have been caused by the slicing (“cytokine storm”) | |||
| Media | (D)MEM, Ham’s F-12, RPMI, Medium 199, EBSS, HBSS | • Several protocols use the same culture media during the procedure | |
| • Additional supplements may include CaCl2, MgSO4, KCl, NaCl, NaH2PO4, glucose, NaHCO3, HEPES, penicillin/streptomycin, insulin, hydrocortisone, retinyl acetate, gentamicin, or Amphotericin B | |||
| • The media needs to be optimized depending on the modeled disease | |||
| Serum | • None | • Most protocols do not add serum to cultured PCLS because it might alter cell proliferation | |
| • 0.1% (48) | |||
| • 10% (72) | |||
| Length | Minutes to a few hours | • Earlier studies used only short incubation times to study metabolites | |
| 1–7 d | • Most current PCLS models have shown viability across the different cell types for up to 7 d. Most of the discussed models use durations within this range | ||
| >21 d (68, 80) | • Few studies have reported PCLS culture for up to 28 d. More recently, embedding in hydrogels was shown to extend culture times to >21 d |
Definition of abbreviations: CLDs = chronic lung diseases; DMEM = Dulbecco’s modified Eagle medium; EBSS = Earle’s balanced salt solution; HBSS = Hanks’ balanced salt solution; PCLS = precision-cut lung slices.
The nomenclature used for lung tissue slices has varied somewhat over time. Initially, the term “PCLS” was used (29), and later these slices were referred to as PCLuS (30), precision-cut pulmonary slices (31), lung tissue slices (32), organotypic lung slices (33), and, more recently, three-dimensional (3D) lung tissue cultures (15, 34). Here, we will use the initial term describing the basic methodology: PCLS.
PCLS: A Suitable Tool for Disease Modeling
Our understanding of molecular, cellular and extracellular interactions in two-dimensional cultures is limited, and although complexity can be increased with further modifications using (3D) co-culture and (bio) artificial matrices (35), one major advantage offered by PCLS is the ability to study the structure and function of the lung in its native 3D environment, and thus natural interactions between cells, molecules, and the extracellular matrix (ECM) ex vivo. PCLS generation specifically from human tissue has several important advantages: 1) PCLS allow for paired analysis of several treatments in the same patient, 2) PCLS can be generated from different areas within the same lung to represent tissue (and thus disease) heterogeneity, 3) generation of PCLS from tissue explants from healthy donors can be applied for ex vivo modeling of disease initiation and progression through treatment with known contributing stressors, cytokines, enzymes, growth factors, or environmental agents and pollutants; and 4) PCLS derived from lungs of patients with CLD enable analysis of disease-relevant mechanisms, including the effects of different therapeutic agents depending on disease status (summarized in Figure 2). Below, we discuss how these advantages of PCLS have recently been applied to study COPD, IPF, and lung cancer (see also Table 2).
Figure 2.
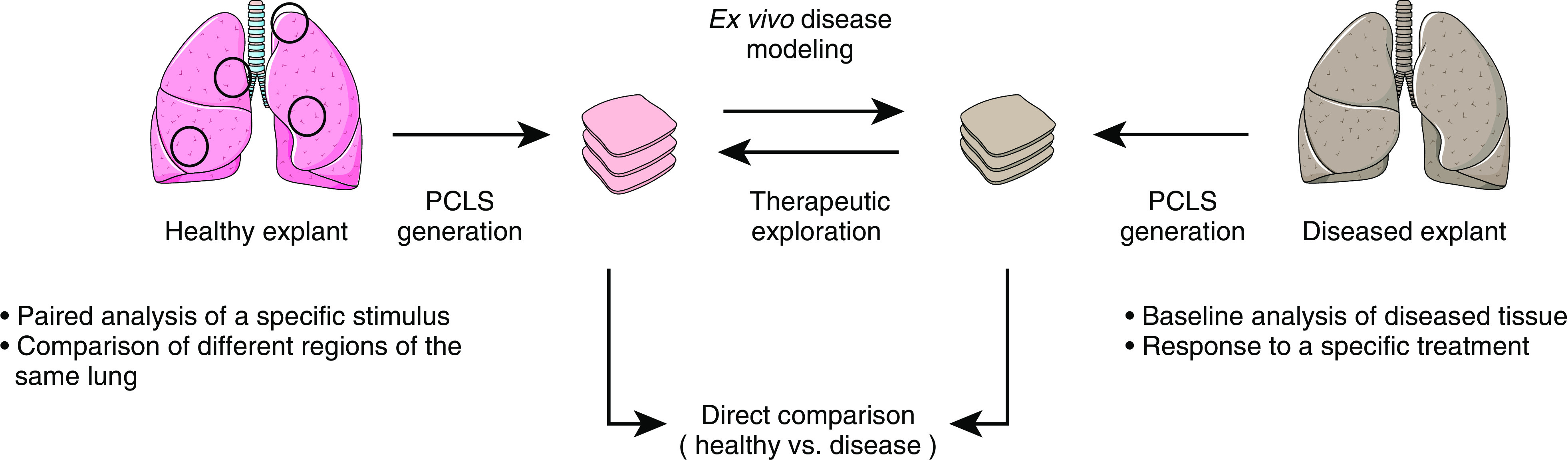
Schematic outline of PCLS use for disease modeling and drug discovery. Paired analysis of slices obtained from the same lung region allows exploration of stimulus-specific effects (open circles). Furthermore, this effect can be explored in different regions of the same lung to assess patient-specific tissue heterogeneity and responses to a certain stimulus. PCLS generation from healthy explants and diseased tissue can reveal anatomic differences in the molecular interactions that occur within the microenvironment of the lung. Disease modeling of healthy tissue can be achieved ex vivo by mimicking disease characteristics. Ultimately, disease models from natively diseased tissue or models developed ex vivo can be used for therapeutic exploration. Images reproduced and modified from Servier Medical Art with permission (http://smart.servier.com/).
Table 2.
Disease Modeling Using Precision-Cut Lung Slices
| Disease | Ex Vivo/In Vivo | Model | Species | Research Focus/Readout | Ref. |
|---|---|---|---|---|---|
| COPD | In vivo | Elastase model | Mouse | Exploration of Wnt-β-catenin–induced repair in emphysema | (15) |
| CS exposure and viral exacerbation | Mouse | PPAR-γ agonists as treatment for H1N1 influenza | (76) | ||
| Influenza A–induced exacerbations | (12) | ||||
| CS exposure | Mouse | Ciliary beating in epithelial cells | (81) | ||
| CS-induced neutrophilia | (82) | ||||
| Guinea pig | Vasoconstriction and relaxation of CS-exposed arteries | (41) | |||
| Human COPD | Human | Exploration of Wnt/β-catenin induction as regenerative therapy | (15) | ||
| Ex vivo | Elastase | Mouse | Ex vivo modeling of COPD | (38) | |
| CS exposure | Mouse | Assessment of ex vivo exposure to CS | (83) | ||
| CS condensate | Rodent, rhesus, and human | Characterization of PCLS response to CS condensate treatment | (83) | ||
| Polyinosinic-polycytidylic acid | Human | Inflammatory cytokines’ response to Toll-like receptor 3 activation and effect on small airway mechanics | (42) | ||
| LPS treatment | Human | Immunosuppressive therapy in human PCLS | (16) | ||
| IPF | In vivo | Bleomycin | Mouse | Effect of caffeine on extracellular matrix in a bleomycin model of fibrosis | (46) |
| Rat | Study of collagen tissue turnover in fibrotic rat lung tissue | (84) | |||
| Transgenic TGF-β | Mouse | Targeting of collagen deposition in CC10 promoter–driven TGF-β activation fibrosis | (85) | ||
| Human IPF | Human | Exploration of PI3 kinase/mTOR inhibitor for antifibrotic therapy | (45) | ||
| Ex vivo | Bleomycin | Rat | Study of the interaction of caveolin-1 and CD147 | (86) | |
| TGF-β + CdCl2 | Rat | Characterization of an ex vivo model by immunohistological assessments | (87) | ||
| Fibrosis cocktail | Human | Modeling early fibrosis-like changes in normal human PCLS | (48) | ||
| Cancer | In vivo | KRAS mutation | Mouse | LSL-KrasG12D/+; Lkb1fl/fl (Kras; Lkb1 as a lung cancer model) | (60) |
| Nanoparticle treatment on PCLS from mouse tumor lung tissue | (58) | ||||
| Exploration of heterogeneity in lung cancer | (61) | ||||
| Tumor resection | Human | Oligonucleotide–nanoparticle complexes to target telomerase activity in resection of non–small cell lung cancer tumors | (62) | ||
| Ex vivo nanoparticle delivery on human PCLS derived from adenocarcinoma and secondary lung tumor resections | (58) | ||||
| Tumor resection | Human | Mechanism of T-cell infiltration in PCLS derived from lung tumor resections | (39) | ||
| Other models | Ex vivo | Anthrax | Human | Characterization of the immune response to infection with Bacillus anthracis spores | (75) |
| Tuberculosis | Human | Evaluation of immune response to different strains of M. tuberculosis infection ex vivo | (72) | ||
| Influenza virus | Human | Effects of CS extract on the response of PCLS to viral stimuli | (78) | ||
| HRV virus | Human | Response of PCLS to HRV infection | (88) | ||
| Silica-induced inflammation | Mouse | Exploration of the role of macrophages in early lung inflammation through co-culture of immortalized macrophages with lung slices exposed to silica | (33) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; CS = cigarette smoke; HRV = human rhinovirus; IPF = idiopathic pulmonary fibrosis; M. tuberculosis = Mycobacterium tuberculosis; PPAR-γ = peroxisome proliferator-active receptor γ; TGF-β = transforming growth factor-β.
COPD
COPD is a lethal CLD that is among the top three leading causes of death globally (3). It is largely caused by inhaled toxins and environmental stressors, as well as genetic susceptibility (3). COPD is characterized by inflammation of the respiratory tract, small airway disease, and parenchymal tissue destruction (3). Changes in the parenchymal regions surrounding the small airways play an important role in COPD, with the loss of elastin fibers and other ECM components resulting in progressive airspace enlargement and loss of functional surface area, called emphysema (11).
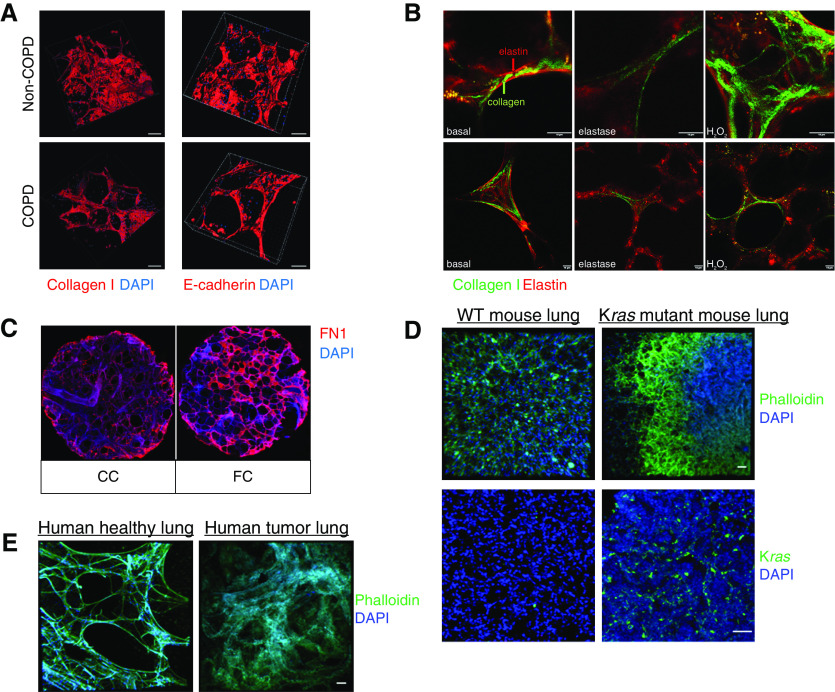
Most of the existing animal models of COPD are driven by inflammation, largely induced in young animals by cigarette smoke exposure or elastase treatment in vivo. These models resemble some, but not all, aspects of human COPD (9, 10). PCLS can be generated from these animals to explore molecular and cellular interactions in this disease, and reduce the number of animals required (Table 2) (12, 15, 36, 37). More recently, ex vivo elastase treatment on PCLS was shown to result in effects similar to those observed with in vivo elastase models, such as a loss of structural integrity of elastin and collagen fibers, and airspace destruction (Figure 3) (38). Because this process is highly dependent on neutrophilic inflammation, these data suggest that the exogenous elastase was sufficient to elicit the observed response. This enzyme has a rather short half-life, so it is possible that neutrophils present in the slices further contribute to the observed inflammation. This is particularly important because one potential limitation of PCLS ex vivo cultures compared with in vivo models is the absence of potential influx/homing of immune cells. Impaired immune cell signaling is involved in the development of progression of most CLDs, and current PCLS cultures do not allow for recruitment of immune cells in a manner that is known to occur in disease. Importantly, however, different studies have also taken advantage of this situation to investigate cellular processes that are independent of immune cell homing, and/or to specifically add immune cells to the ex vivo PCLS culture system (39).
Figure 3.
PCLS disease modeling of COPD, IPF, and lung cancer. (A) Three-dimensional reconstruction of collagen I and E-cadherin staining on PCLS generated from healthy and COPD human explants (15). Scale bars: 100 μm. (B) Collagen I and elastin fibers in an ex vivo elastase COPD disease model in mouse PCLS (38). Scale bars: 10 μm. (C) Extracellular matrix deposition of fibronectin in PCLS treated with a fibrotic cocktail (FC) to model early fibrosis-like changes (48). Scale bar: 1 mm. (D) PCLS immunostained against phalloidin and Kras. PCLS were obtained from the mouse KRAS model (58). Scale bars: 50 μm. (E) Structural differences between tumor and tumor-free regions of PCLS generated from human explants. Scale bars: 50 μm. Reprinted by permission from References 15, 38, 48, and 58. CC = control cocktail; FN1 = fibronectin; WT = wild-type.
To further understand COPD development, investigators have applied specific growth factors, cytokines, or environmental stressors. LPS treatment was found to induce the expression of IL-1α and TNF-α in human PCLS in a dose-dependent manner with minimal changes in tissue viability (16). Cigarette smoke condensate has also been shown to induce specific COPD-relevant outcomes, such as vessel and airway contractility, in rodent, rhesus monkey, and human PCLS (40, 41). Also, activation of the Toll-like receptor 3 by polyinosinic-polycytidylic acid was found to stimulate cytokine secretion in human PCLS (42). The combined application of additional disease-relevant stimuli and environmental stressors on human PCLS may provide a further opportunity to understand the potential role of these stimuli in the human lung, and better reflect the complexity of COPD pathogenesis.
Importantly, diseased PCLS from patients with COPD have been used to evaluate the pathomechanisms of this disease and to identify and validate novel potential therapies (15). We first described the generation, characterization, and application of patient-derived PCLS in a study focused on potential mechanisms for lung repair in COPD. Specifically, pharmacological activation of Wnt/β-catenin signaling in PCLS from patients with COPD led to the initiation of alveolar epithelial cell repair ex vivo (15). In a follow-up study, PCLS were used to demonstrate that pharmacological induction of expression of the Wnt receptor frizzled 4 further induced Wnt/β-catenin signaling and promoted elastogenesis, thus providing additional evidence for the feasibility of targeting the Wnt/β-catenin pathway as a potential regenerative therapy for COPD (43). More recently, small-airway hyperresponsiveness was demonstrated in PCLS obtained from patients with COPD (44). Altogether, PCLS have been successfully applied to investigate the molecular drivers and clinically relevant features of COPD and emphysema, as well as potential novel therapeutic options to initiate tissue repair upon loss of lung structure and function.
IPF
IPF is a lethal disease with a complex etiology characterized by impaired function of several cellular compartments, including lung epithelial injury and reprogramming, immune cell dysfunction, and (myo-)fibroblast activation, all of which lead to pathological expression and deposition of ECM and lung scarring (2). Although several potential therapeutic targets have been discovered over the last few years, many of these have failed in clinical trials even though they showed efficacy in animal models. Human-derived PCLS thus might be helpful for further preclinical investigation and validation. PCLS obtained from explants from patients with IPF have been used to explore several disease mechanisms and therapeutic targets (45, 46). Mercer and colleagues applied PCLS to elucidate the appropriate concentration of a novel PI3K inhibitor, GSK2126458, in PCLS derived from IPF tissue (45). They reported that targeting PI3K/mTOR led to reduced expression of the collagen formation marker P1NP (pro-collagen 1 amino-terminal peptide), thus highlighting GSK2126458 as a new potential drug compound for IPF. More recently, it was found that the role of the PI3K/mTOR pathway in IPF is exerted through myofibroblasts, and additional novel inhibitors of the PI3K/mTOR pathway were tested in human PCLS (47). Although these studies explored the potential of using end-stage IPF tissue to test novel compounds, the rarity of IPF explants and the difficulty of homogeneously filling heavily fibrotic and scarred tissue with agarose before slicing present major challenges (20). Furthermore, because this tissue comes from explanted lungs, it limits the scope of studies on end-stage disease pathomechanisms, which may differ from those observed during disease onset and early progression, a time window that is largely considered optimal for targeting potential reversal of the disease. A recently developed ex vivo human model of PF exhibited the ability to recapitulate early fibrosis-like changes in nonfibrotic PCLS, thus providing a complementary approach to study early fibrogenesis (48). In this model, fibrotic changes were induced by the use of a fibrotic cocktail (FC) consisting of potent growth factors, inflammatory cytokines, and signaling molecules known to be elevated in IPF. Within up to 5 days, the FC treatment led to the expression of well-known fibrotic markers and proinflammatory cytokines, along with deposition of the ECM components collagen I and fibronectin (Figure 3C). In addition, phenotypic markers of putative distal lung progenitor cell populations such as SFTPC and HOPX (Homeobox Only Protein X) genes were reduced, mimicking the loss and/or reduction of these markers seen in patients with IPF (49). The FC model has been used to further validate the effects of the two U.S. Food and Drug Administration–approved drugs for IPF, nintedanib and pirfenidone (34). Notably, treatment with nintedanib, but not pirfenidone, was shown to increase the expression of alveolar epithelial markers that had originally been reduced by FC (34). This indicates that these two drugs have different modes of action, and highlights a major potential application of human PCLS in precision medicine, as future studies may be able to elucidate drug responses in PCLS from individual patients. In a more recent study, the same FC model was used to explore the effect of histone deacetylase EP300 inhibition as a potential novel target for IPF treatment (50). Inhibition of EP300 was found to attenuate the fibrotic characteristics of primary patient-derived fibroblasts, experimental lung fibrosis in vivo, and in the FC PCLS model ex vivo. Similarly, Roach and colleagues explored an alternative ex vivo culture of 2-mm3 parenchymal lung pieces for up to 7 days in the presence of TGF-β (transforming growth factor-β) (51). Although the thickness of the tissue might limit the viability of the tissue or induce hypoxia, they showed that TGF-β–induced changes in myofibroblast marker and ECM expression could be attenuated by inhibiting the profibrotic KCa 3.1 ion channels (51). To date, the changes shown in most studies have been based primarily on stimulation with soluble factors. Future studies altering the structure and microenvironment of the PCLS, including changes in stretch and stiffness, need to be implemented to advance PF models (26).
Lung Cancer
Lung cancer is one of the most frequently diagnosed cancers and represents the main cause of cancer-related deaths worldwide (52). Lung cancer can result from exposure to chemical agents, environmental toxins, and/or genetic mutations (53, 54). Currently, the only therapeutic option available is resection followed by chemotherapy, which is accompanied by high relapse rates as well as the development of chemotherapy resistance. It was reported that 30–55% of patients with non–small cell lung cancer who undergo resection have reoccurring tumors, resulting in mortality (55). Several different animal models of cancer have been used to test molecular mechanisms and therapeutics, such as transgenic mice (e.g., KRAS), chemically induced carcinoma, and adenoviral KRAS mouse models (55, 56). However, as is the case with other CLDs, these models do not reflect the inherent genetic and cellular complexity of cancerogenesis in humans (56). In addition to being a primary site of cancer development, the lung is often affected by metastasis from other cancers. As such, PCLS are also relevant for studies investigating the lung environment for the formation of metastasis. Up to now, the use of PCLS in cancer research has been limited to a few aspects of the disease, such as inflammatory processes in rodents and humans (57, 58). Vaira and colleagues profiled sliced tumor specimens; however, the slices used in their study were produced without agarose filling, and their thickness varied between 300 μm and 500 μm (59), making it difficult to compare with other models. Recently, Davies and colleagues characterized an ex vivo PCLS model of lung adenocarcinoma using immunohistology and gene expression of established cancer markers (60) (Table 2). Using this PCLS disease model, Narhi and colleagues explored combination therapy for adenocarcinoma and demonstrated the ability to examine the heterogeneity in tumor progression within PCLS, as different oncogenic signaling pathways were activated in neighboring regions in the parenchyma of the lung (61). This study highlights the importance of using combination therapy in the treatment of heterogeneous cancers, and might serve as an example to study novel therapeutic approaches, including combination therapy, in other CLDs.
Cues from the tumor microenvironment can also be recapitulated in PCLS. For example, it has been described that T cells lose their antitumor surveillance functions because of their inability to penetrate the tumor’s stromal tissue. It was believed that in less dense regions, T-cell motility is mediated by integrin binding. However, in a recent study, live imaging of human PCLS from tumor resections revealed that T-cell motility is mediated by chemokines (39).
One major advantage of PCLS is that they potentially can be used to investigate novel drug delivery vehicles and approaches. In cancer, PCLS has been used to explore the therapeutic application of two different types of nanoparticles—mesoporous silica nanoparticles and oligonucleotide–nanoparticle complexes—as novel delivery vehicles. Using PCLS derived from explanted human lung cancers and the KRAS mouse model, two studies were able to demonstrate the feasibility of nanoparticle-driven therapies to selectively induce apoptosis in tumor regions (58, 62). Given the recent advances in exogenous nanoparticle generation and modification (63), as well as an emerging interest in endogenously formed, cell-derived vesicles (e.g., microvesicles and exosomes) as mediators for cell–cell communication (64, 65), PCLS offer the potential to further delineate cell-specific uptake and responses in disease-relevant 3D multicellular tissue settings.
To date, no ex vivo PCLS models for lung cancer initiation and development have been reported. The generation of this kind of model most likely would be based on the introduction of genetic mutations ex vivo in addition to chemical carcinogens and other secondary hits. Another compelling application of PCLS would be the generation of PCLS from individual patients undergoing lung tumor resection. PCLS from these tissues could be used to predict potential tumor-cell resistance and test novel therapeutic agents. Furthermore, PCLS from adjacent tumor-free regions of tissue removed during tumor resections may contain molecular cues with respect to the susceptibility of these tissues for relapse or disease progression.
(Pre)clinical Impact, Challenges, and Future Opportunities
The most recent developments in the generation, characterization, and culture of murine and human PCLS have created several new opportunities to enhance our understanding and treatment of CLDs, including COPD, IPF, and lung cancer. A high proportion of novel therapies fail in clinical trials despite their evaluation in animal models, and there is an urgent need for better and more clinically relevant translational approaches for drug discovery and validation (66, 67). PCLS provide a novel and exciting new tool in this area, particularly when generated from human lung tissue. PCLS can be of value for a variety of potential (pre)clinical applications, including identification of novel pathomechanisms and targets, testing of novel drug delivery options (e.g., nanoparticles and vesicles), discovery and validation of novel drugs and compounds, and assessment of individual side effects and treatment responses (Figure 4).
Figure 4.
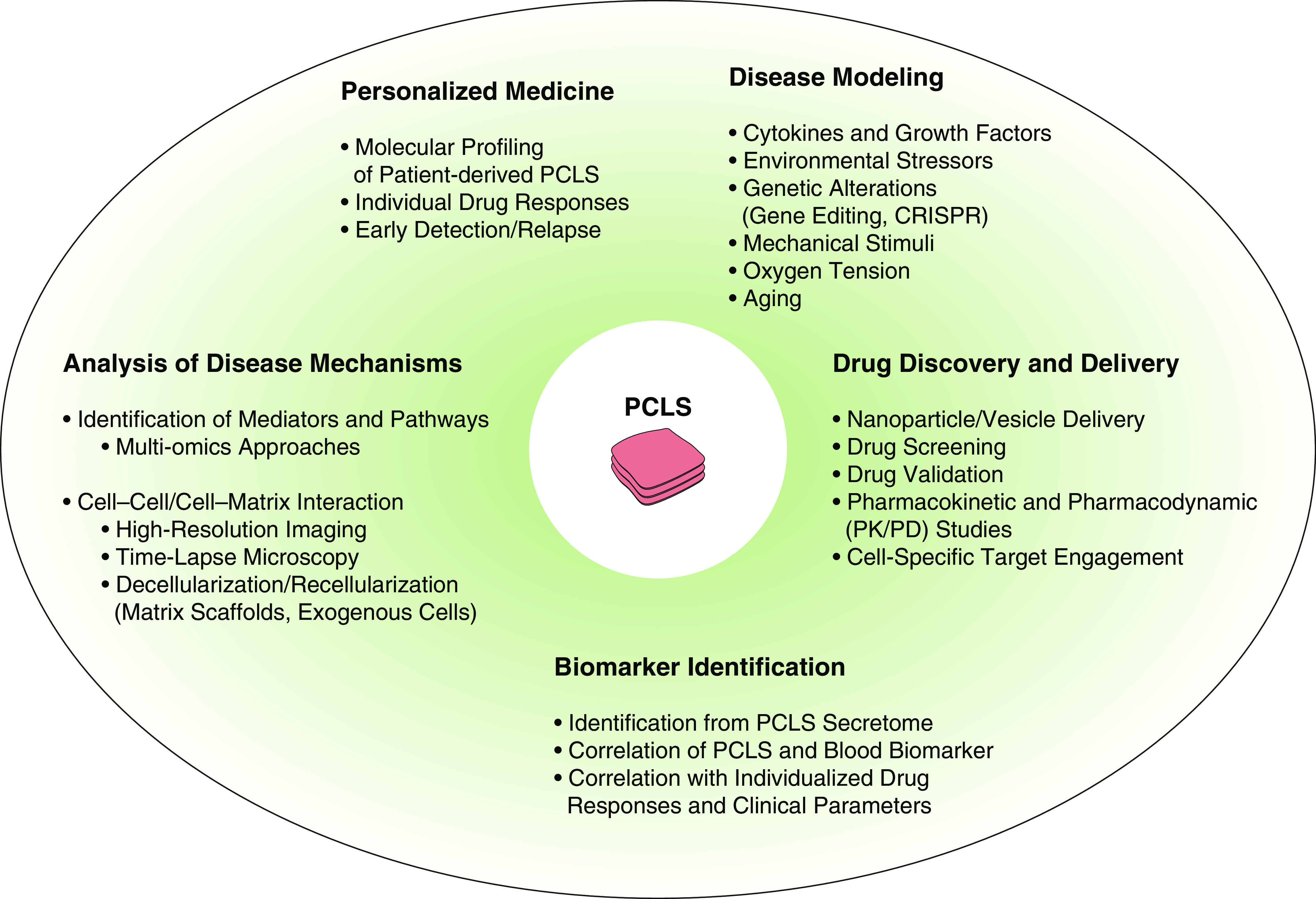
PCLS applications and approaches: current strategies and future opportunities for disease modeling and drug discovery. PCLS may be of value for a variety of (pre)clinical applications, including identification of novel pathomechanisms and targets using novel methodologies and imaging techniques, testing of novel drug delivery options (e.g., nanoparticles and vesicles), discovery and validation of novel drugs and compounds, and assessment of individual side effects and treatment responses. PD = pharmacodynamic; PK = pharmacokinetic.
To achieve these aims, however, several challenges need to be addressed:
1. Human lung tissue suitable for slicing is not easily available at all research institutions, and acquisition of such tissue remains challenging owing to different clinical and regulatory frameworks. Therefore, there is a need to expand tissue availability for research by means of tissue-sharing programs and technologies, as well as the development of standardized workflows among clinicians, surgeons, pathologists, and researchers.
2. Although PCLS undoubtedly offer the major advantage of enabling studies of complex cellular and structural architecture in living human tissue, standardization and improvement of (medium to high-throughput) cultures, analysis tools, and automation conditions remain challenging, and a concerted effort by the community to advance our knowledge and share data will be essential to improve the suitability and reliability of PCLS. In particular, limited PCLS culture times are hampering the field, and it is important to develop approaches to prolong PCLS viability, such as embedding PCLS in hydrogels (68). With respect to analysis tools, continuous advances in microscopy and deep-tissue imaging technologies, as well as single-cell approaches using PCLS, are enabling high temporal and spatial resolution of cellular and molecular mechanisms relevant to CLDs. This is an emerging field with the potential to tremendously enhance the applicability of PCLS in the future, as such studies may allow us to study the function of healthy or impaired human cells in real time in response to a diverse range of treatments.
3. Although promising PCLS disease models have been established, further improvements and modifications of the right “ingredients” to mimic disease are needed. Along these lines, it is important to consider that aging is one of the major risk factors for CLDs (69, 70), and hallmarks of aging need to be integrated as measures within PCLS. One such hallmark, cellular senescence (71), was recently shown to be present in PCLS from an experimental lung fibrosis model, and senolytics were successfully applied in this model to target senescent cells and attenuate fibrosis (70). These approaches need to be further expanded for use in human PCLS (from individuals of different ages) and additional hallmarks need to be investigated.
4. To effectively use PCLS as a bridge to (pre)clinical drug discovery research and (potentially) clinical trials, clinically relevant and quantifiable outcome measures for future studies need to be developed. Ideally, these studies would include analyses of disease-defining elements to elucidate potential drug mechanisms and target engagement, discovery of novel biomarkers, and validation of already established biomarkers. PCLS can be applied to characterize potential biomarkers, including freely secreted or vesicle-bound proteins, nucleic acids, and metabolites, which are produced within the lung tissue and are subsequently released and found in the blood of patients.
Overall, based on encouraging recent findings, these next steps will significantly advance the potential of PCLS as novel clinically relevant translational models, and thus guide the identification and implementation of novel therapies for patients with CLDs.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2019-0276TR on January 28, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc. 2014;11:404–406. doi: 10.1513/AnnalsATS.201311-405PS. [DOI] [PubMed] [Google Scholar]
- 2.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 3.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- 4.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 5.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. CAPACITY Study Group. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 6.Raghu G, Selman M. Nintedanib and pirfenidone: new antifibrotic treatments indicated for idiopathic pulmonary fibrosis offer hopes and raises questions. Am J Respir Crit Care Med. 2015;191:252–254. doi: 10.1164/rccm.201411-2044ED. [DOI] [PubMed] [Google Scholar]
- 7.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore BB, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol. 2013;49:167–179. doi: 10.1165/rcmb.2013-0094TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2008;295:L1–L15. doi: 10.1152/ajplung.90200.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones B, Donovan C, Liu G, Gomez HM, Chimankar V, Harrison CL, et al. Animal models of COPD: what do they tell us? Respirology. 2017;22:21–32. doi: 10.1111/resp.12908. [DOI] [PubMed] [Google Scholar]
- 11.Bidan CM, Veldsink AC, Meurs H, Gosens R. Airway and extracellular matrix mechanics in COPD. Front Physiol. 2015;6:346. doi: 10.3389/fphys.2015.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan C, Seow HJ, Bourke JE, Vlahos R. Influenza A virus infection and cigarette smoke impair bronchodilator responsiveness to β-adrenoceptor agonists in mouse lung. Clin Sci (Lond) 2016;130:829–837. doi: 10.1042/CS20160093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher RL, Smith MS, Hasal SJ, Hasal KS, Gandolfi AJ, Brendel K. The use of human lung slices in toxicology. Hum Exp Toxicol. 1994;13:466–471. doi: 10.1177/096032719401300703. [DOI] [PubMed] [Google Scholar]
- 14.Liberati TA, Randle MR, Toth LA. In vitro lung slices: a powerful approach for assessment of lung pathophysiology. Expert Rev Mol Diagn. 2010;10:501–508. doi: 10.1586/erm.10.21. [DOI] [PubMed] [Google Scholar]
- 15.Uhl FE, Vierkotten S, Wagner DE, Burgstaller G, Costa R, Koch I, et al. Preclinical validation and imaging of Wnt-induced repair in human 3D lung tissue cultures. Eur Respir J. 2015;46:1150–1166. doi: 10.1183/09031936.00183214. [DOI] [PubMed] [Google Scholar]
- 16.Switalla S, Lauenstein L, Prenzler F, Knothe S, Förster C, Fieguth HG, et al. Natural innate cytokine response to immunomodulators and adjuvants in human precision-cut lung slices. Toxicol Appl Pharmacol. 2010;246:107–115. doi: 10.1016/j.taap.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Akram KM, Yates LL, Mongey R, Rothery S, Gaboriau DCA, Sanderson J, et al. Live imaging of alveologenesis in precision-cut lung slices reveals dynamic epithelial cell behaviour. Nat Commun. 2019;10:1178. doi: 10.1038/s41467-019-09067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgstaller G, Sengupta A, Vierkotten S, Preissler G, Lindner M, Behr J, et al. Distinct niches within the extracellular matrix dictate fibroblast function in (cell free) 3D lung tissue cultures. Am J Physiol Lung Cell Mol Physiol. 2018;314:L708–L723. doi: 10.1152/ajplung.00408.2017. [DOI] [PubMed] [Google Scholar]
- 19.Burgstaller G, Vierkotten S, Lindner M, Königshoff M, Eickelberg O. Multidimensional immunolabeling and 4D time-lapse imaging of vital ex vivo lung tissue. Am J Physiol Lung Cell Mol Physiol. 2015;309:L323–L332. doi: 10.1152/ajplung.00061.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerckens M, Alsafadi HN, Wagner DE, Lindner M, Burgstaller G, Königshoff M. Generation of human 3D lung tissue cultures (3D-LTCs) for disease modeling. J Vis Exp. 2019;(144) doi: 10.3791/58437. DOI: 10.3791/58437. [DOI] [PubMed] [Google Scholar]
- 21.Lyons-Cohen MR, Thomas SY, Cook DN, Nakano H. Precision-cut mouse lung slices to visualize live pulmonary dendritic cells. J Vis Exp. 2017;(122) doi: 10.3791/55465. DOI: 10.3791/55465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagaraj AS, Bao J, Hemmes A, Machado M, Närhi K, Verschuren EW. Establishment and analysis of tumor slice explants as a prerequisite for diagnostic testing. J Vis Exp. 2018;(141) doi: 10.3791/58569. DOI: 10.3791/58569. [DOI] [PubMed] [Google Scholar]
- 23.Neuhaus V, Danov O, Konzok S, Obernolte H, Dehmel S, Braubach P, et al. Assessment of the cytotoxic and immunomodulatory effects of substances in human precision-cut lung slices. J Vis Exp. 2018;(135) doi: 10.3791/57042. DOI: 10.3791/57042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dassow C, Wiechert L, Martin C, Schumann S, Müller-Newen G, Pack O, et al. Biaxial distension of precision-cut lung slices. J Appl Physiol (1985) 2010;108:713–721. doi: 10.1152/japplphysiol.00229.2009. [DOI] [PubMed] [Google Scholar]
- 25.Davidovich N, Huang J, Margulies SS. Reproducible uniform equibiaxial stretch of precision-cut lung slices. Am J Physiol Lung Cell Mol Physiol. 2013;304:L210–L220. doi: 10.1152/ajplung.00224.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Froese AR, Shimbori C, Bellaye P-S, Inman M, Obex S, Fatima S, et al. Stretch-induced activation of transforming growth factor-β1 in pulmonary fibrosis. Am J Respir Crit Care Med. 2016;194:84–96. doi: 10.1164/rccm.201508-1638OC. [DOI] [PubMed] [Google Scholar]
- 27.Davidovich N, Chhour P, Margulies SS. Uses of remnant human lung tissue for mechanical stretch studies. Cell Mol Bioeng. 2013;6:175–182. doi: 10.1007/s12195-012-0263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song MJ, Davidovich N, Lawrence GG, Margulies SS. Superoxide mediates tight junction complex dissociation in cyclically stretched lung slices. J Biomech. 2016;49:1330–1335. doi: 10.1016/j.jbiomech.2015.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefaniak MS, Krumdiek CL, Spall RD, Gandolfi AJ, Brendel K. Biochemical and histological characterization of agar-filled precision-cut rat lung slices in dynamic organ culture as an in vitro tool. In Vitro Toxicol. 1992;5:7–19. [Google Scholar]
- 30.Sauer UG, Vogel S, Aumann A, Hess A, Kolle SN, Ma-Hock L, et al. Applicability of rat precision-cut lung slices in evaluating nanomaterial cytotoxicity, apoptosis, oxidative stress, and inflammation. Toxicol Appl Pharmacol. 2014;276:1–20. doi: 10.1016/j.taap.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Westra IM, Pham BT, Groothuis GMM, Olinga P. Evaluation of fibrosis in precision-cut tissue slices. Xenobiotica. 2013;43:98–112. doi: 10.3109/00498254.2012.723151. [DOI] [PubMed] [Google Scholar]
- 32.Freeman BA, O’Neil JJ. Tissue slices in the study of lung metabolism and toxicology. Environ Health Perspect. 1984;56:51–60. doi: 10.1289/ehp.845651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmann F, Bläsche R, Kasper M, Barth K. A co-culture system with an organotypic lung slice and an immortal alveolar macrophage cell line to quantify silica-induced inflammation. PLoS One. 2015;10:e0117056. doi: 10.1371/journal.pone.0117056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehmann M, Buhl L, Alsafadi HN, Klee S, Hermann S, Mutze K, et al. Differential effects of Nintedanib and Pirfenidone on lung alveolar epithelial cell function in ex vivo murine and human lung tissue cultures of pulmonary fibrosis. Respir Res. 2018;19:175. doi: 10.1186/s12931-018-0876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiemstra PS, Tetley TD, Janes SM. Airway and alveolar epithelial cells in culture. Eur Respir J. 2019;54:1900742. doi: 10.1183/13993003.00742-2019. [DOI] [PubMed] [Google Scholar]
- 36.Kistemaker LEM, Oenema TA, Baarsma HA, Bos IST, Schmidt M, Facchinetti F, et al. The PDE4 inhibitor CHF-6001 and LAMAs inhibit bronchoconstriction-induced remodeling in lung slices. Am J Physiol Lung Cell Mol Physiol. 2017;313:L507–L515. doi: 10.1152/ajplung.00069.2017. [DOI] [PubMed] [Google Scholar]
- 37.Wohlsen A, Hirrle A, Tenor H, Marx D, Beume R. Effect of cyclic AMP-elevating agents on airway ciliary beat frequency in central and lateral airways in rat precision-cut lung slices. Eur J Pharmacol. 2010;635:177–183. doi: 10.1016/j.ejphar.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Van Dijk EM, Culha S, Menzen MH, Bidan CM, Gosens R. Elastase-induced parenchymal disruption and airway hyper responsiveness in mouse precision cut lung slices: toward an ex vivo COPD model. Front Physiol. 2017;7:657. doi: 10.3389/fphys.2016.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean M-C, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreno L, Perez-Vizcaino F, Harrington L, Faro R, Sturton G, Barnes PJ, et al. Pharmacology of airways and vessels in lung slices in situ: role of endogenous dilator hormones. Respir Res. 2006;7:111. doi: 10.1186/1465-9921-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright JL, Churg A. Short-term exposure to cigarette smoke induces endothelial dysfunction in small intrapulmonary arteries: analysis using guinea pig precision cut lung slices. J Appl Physiol (1985) 2008;104:1462–1469. doi: 10.1152/japplphysiol.00520.2007. [DOI] [PubMed] [Google Scholar]
- 42.Cooper PR, Lamb R, Day ND, Branigan PJ, Kajekar R, San Mateo L, et al. TLR3 activation stimulates cytokine secretion without altering agonist-induced human small airway contraction or relaxation. Am J Physiol Lung Cell Mol Physiol. 2009;297:L530–L537. doi: 10.1152/ajplung.00133.2009. [DOI] [PubMed] [Google Scholar]
- 43.Skronska-Wasek W, Mutze K, Baarsma HA, Bracke KR, Alsafadi HN, Lehmann M, et al. Reduced frizzled receptor 4 expression prevents WNT/β-catenin-driven alveolar lung repair in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196:172–185. doi: 10.1164/rccm.201605-0904OC. [DOI] [PubMed] [Google Scholar]
- 44.Maarsingh H, Bidan CM, Brook BS, Zuidhof AB, Elzinga CRS, Smit M, et al. Small airway hyperresponsiveness in COPD: relationship between structure and function in lung slices. Am J Physiol Lung Cell Mol Physiol. 2019;316:L537–L546. doi: 10.1152/ajplung.00325.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mercer PF, Woodcock HV, Eley JD, Platé M, Sulikowski MG, Durrenberger PF, et al. Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax. 2016;71:701–711. doi: 10.1136/thoraxjnl-2015-207429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tatler AL, Barnes J, Habgood A, Goodwin A, McAnulty RJ, Jenkins G. Caffeine inhibits TGFβ activation in epithelial cells, interrupts fibroblast responses to TGFβ, and reduces established fibrosis in ex vivo precision-cut lung slices. Thorax. 2016;71:565–567. doi: 10.1136/thoraxjnl-2015-208215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodcock HV, Eley JD, Guillotin D, Platé M, Nanthakumar CB, Martufi M, et al. The mTORC1/4E-BP1 axis represents a critical signaling node during fibrogenesis. Nat Commun. 2019;10:6. doi: 10.1038/s41467-018-07858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alsafadi HN, Staab-Weijnitz CA, Lehmann M, Lindner M, Peschel B, Königshoff M, et al. An ex vivo model to induce early fibrosis-like changes in human precision-cut lung slices. Am J Physiol Lung Cell Mol Physiol. 2017;312:L896–L902. doi: 10.1152/ajplung.00084.2017. [DOI] [PubMed] [Google Scholar]
- 49.Jain R, Barkauskas CE, Takeda N, Bowie EJ, Aghajanian H, Wang Q, et al. Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nat Commun. 2015;6:6727. doi: 10.1038/ncomms7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubio K, Singh I, Dobersch S, Sarvari P, Günther S, Cordero J, et al. Inactivation of nuclear histone deacetylases by EP300 disrupts the MiCEE complex in idiopathic pulmonary fibrosis. Nat Commun. 2019;10:2229. doi: 10.1038/s41467-019-10066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roach KM, Sutcliffe A, Matthews L, Elliott G, Newby C, Amrani Y, et al. A model of human lung fibrogenesis for the assessment of anti-fibrotic strategies in idiopathic pulmonary fibrosis. Sci Rep. 2018;8:342. doi: 10.1038/s41598-017-18555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Groot PM, Wu CC, Carter BW, Munden RF. The epidemiology of lung cancer. Transl Lung Cancer Res. 2018;7:220–233. doi: 10.21037/tlcr.2018.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park K-S, Liang M-C, Raiser DM, Zamponi R, Roach RR, Curtis SJ, et al. Characterization of the cell of origin for small cell lung cancer. Cell Cycle. 2011;10:2806–2815. doi: 10.4161/cc.10.16.17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, et al. Non-small-cell lung cancer. Nat Rev Dis Primers. 2015;1:15009. doi: 10.1038/nrdp.2015.9. [DOI] [PubMed] [Google Scholar]
- 55.Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014;3:242–249. doi: 10.3978/j.issn.2218-6751.2013.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meuwissen R, Berns A. Mouse models for human lung cancer. Genes Dev. 2005;19:643–664. doi: 10.1101/gad.1284505. [DOI] [PubMed] [Google Scholar]
- 57.Behrsing HP, Furniss MJ, Davis M, Tomaszewski JE, Parchment RE. In vitro exposure of precision-cut lung slices to 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole lysylamide dihydrochloride (NSC 710305, Phortress) increases inflammatory cytokine content and tissue damage. Toxicol Sci. 2013;131:470–479. doi: 10.1093/toxsci/kfs319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Rijt SH, Bölükbas DA, Argyo C, Datz S, Lindner M, Eickelberg O, et al. Protease-mediated release of chemotherapeutics from mesoporous silica nanoparticles to ex vivo human and mouse lung tumors. ACS Nano. 2015;9:2377–2389. doi: 10.1021/nn5070343. [DOI] [PubMed] [Google Scholar]
- 59.Vaira V, Fedele G, Pyne S, Fasoli E, Zadra G, Bailey D, et al. Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc Natl Acad Sci USA. 2010;107:8352–8356. doi: 10.1073/pnas.0907676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davies EJ, Dong M, Gutekunst M, Närhi K, van Zoggel HJ, Blom S, et al. Capturing complex tumour biology in vitro: histological and molecular characterisation of precision cut slices. Sci Rep. 2015;5:17187. doi: 10.1038/srep17187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Närhi K, Nagaraj AS, Parri E, Turkki R, van Duijn PW, Hemmes A, et al. Spatial aspects of oncogenic signalling determine the response to combination therapy in slice explants from Kras-driven lung tumours. J Pathol. 2018;245:101–113. doi: 10.1002/path.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong M, Philippi C, Loretz B, Nafee N, Schaefer UF, Friedel G, et al. Tissue slice model of human lung cancer to investigate telomerase inhibition by nanoparticle delivery of antisense 2′-O-methyl-RNA. Int J Pharm. 2011;419:33–42. doi: 10.1016/j.ijpharm.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 63.van Rijt SH, Bein T, Meiners S. Medical nanoparticles for next generation drug delivery to the lungs. Eur Respir J. 2014;44:765–774. doi: 10.1183/09031936.00212813. [DOI] [PubMed] [Google Scholar]
- 64.Martin-Medina A, Lehmann M, Burgy O, Hermann S, Baarsma HA, Wagner DE, et al. Increased extracellular vesicles mediate WNT-5A signaling in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. doi: 10.1164/rccm.201708-1580OC. [online ahead of print] 25 Jul 2018; DOI: 10.1164/rccm.201708-1580OC. [DOI] [PubMed] [Google Scholar]
- 65.Nana-Sinkam SP, Acunzo M, Croce CM, Wang K. Extracellular vesicle biology in the pathogenesis of lung disease. Am J Respir Crit Care Med. 2017;196:1510–1518. doi: 10.1164/rccm.201612-2457PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barnes PJ, Bonini S, Seeger W, Belvisi MG, Ward B, Holmes A. Barriers to new drug development in respiratory disease. Eur Respir J. 2015;45:1197–1207. doi: 10.1183/09031936.00007915. [DOI] [PubMed] [Google Scholar]
- 67.Arrowsmith J, Miller P. Trial watch: phase II and phase III attrition rates 2011-2012. Nat Rev Drug Discov. 2013;12:569. doi: 10.1038/nrd4090. [DOI] [PubMed] [Google Scholar]
- 68.Bailey KE, Pino C, Lennon ML, Lyons A, Jacot JG, Lammers SR, et al. Embedding of precision-cut lung slices in engineered hydrogel biomaterials supports extended ex vivo culture. Am J Respir Cell Mol Biol. 2020;62:14–22. doi: 10.1165/rcmb.2019-0232MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sellarés J, Rojas M. Quercetin in idiopathic pulmonary fibrosis: another brick in the senolytic wall. Am J Respir Cell Mol Biol. 2019;60:3–4. doi: 10.1165/rcmb.2018-0267ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN, et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J. 2017;50:1602367. doi: 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamsanathan S, Alder JK, Sellares J, Rojas M, Gurkar AU, Mora AL. Cellular senescence: the trojan horse in chronic lung diseases. Am J Respir Cell Mol Biol. 2019;61:21–30. doi: 10.1165/rcmb.2018-0410TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carranza-Rosales P, Carranza-Torres IE, Guzmán-Delgado NE, Lozano-Garza G, Villarreal-Treviño L, Molina-Torres C, et al. Modeling tuberculosis pathogenesis through ex vivo lung tissue infection. Tuberculosis (Edinb) 2017;107:126–132. doi: 10.1016/j.tube.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neuhaus V, Schwarz K, Klee A, Seehase S, Förster C, Pfennig O, et al. Functional testing of an inhalable nanoparticle based influenza vaccine using a human precision cut lung slice technique. PLoS One. 2013;8:e71728. doi: 10.1371/journal.pone.0071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Donovan C, Bailey SR, Tran J, Haitsma G, Ibrahim ZA, Foster SR, et al. Rosiglitazone elicits in vitro relaxation in airways and precision cut lung slices from a mouse model of chronic allergic airways disease. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1219–L1228. doi: 10.1152/ajplung.00156.2015. [DOI] [PubMed] [Google Scholar]
- 75.Chakraborty S, Njah K, Pobbati AV, Lim YB, Raju A, Lakshmanan M, et al. Agrin as a mechanotransduction signal regulating YAP through the Hippo pathway. Cell Rep. 2017;18:2464–2479. doi: 10.1016/j.celrep.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 76.Bauer CM, Zavitz CC, Botelho FM, Lambert KN, Brown EG, Mossman KL, et al. Treating viral exacerbations of chronic obstructive pulmonary disease: insights from a mouse model of cigarette smoke and H1N1 influenza infection. PLoS One. 2010;5:e13251. doi: 10.1371/journal.pone.0013251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sturton RG, Trifilieff A, Nicholson AG, Barnes PJ. Pharmacological characterization of indacaterol, a novel once daily inhaled 2 adrenoceptor agonist, on small airways in human and rat precision-cut lung slices. J Pharmacol Exp Ther. 2008;324:270–275. doi: 10.1124/jpet.107.129296. [DOI] [PubMed] [Google Scholar]
- 78.Wu W, Patel KB, Booth JL, Zhang W, Metcalf JP. Cigarette smoke extract suppresses the RIG-I-initiated innate immune response to influenza virus in the human lung. Am J Physiol Lung Cell Mol Physiol. 2011;300:L821–L830. doi: 10.1152/ajplung.00267.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Löfdahl A, Wenglén C, Rydell-Törmänen K, Westergren-Thorsson G, Larsson-Callerfelt A-K. Effects of 5-hydroxytryptamine class 2 receptor antagonists on bronchoconstriction and pulmonary remodeling processes. Am J Pathol. 2018;188:1113–1119. doi: 10.1016/j.ajpath.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 80.Placke ME, Fisher GL. Adult peripheral lung organ culture—a model for respiratory tract toxicology. Toxicol Appl Pharmacol. 1987;90:284–298. doi: 10.1016/0041-008x(87)90336-x. [DOI] [PubMed] [Google Scholar]
- 81.Wyatt TA, Sisson JH, Allen-Gipson DS, McCaskill ML, Boten JA, DeVasure JM, et al. Co-exposure to cigarette smoke and alcohol decreases airway epithelial cell cilia beating in a protein kinase Cε-dependent manner. Am J Pathol. 2012;181:431–440. doi: 10.1016/j.ajpath.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Botelho FM, Bauer CM, Finch D, Nikota JK, Zavitz CC, Kelly A, et al. IL-1α/IL-1R1 expression in chronic obstructive pulmonary disease and mechanistic relevance to smoke-induced neutrophilia in mice. PLoS One. 2011;6:e28457. doi: 10.1371/journal.pone.0028457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Obernolte H, Ritter D, Knebel J, Braubach P, Jonigk D, Warnecke G, et al. Cigarette smoke and cigarette smoke condensate induce inflammation and cytotoxicity in precision-cut lung slices (PCLS) Pneumologie. 2016;70:P42. [Google Scholar]
- 84.Hansen NU, Karsdal MA, Brockbank S, Cruwys S, Rønnow S, Leeming DJ. Tissue turnover of collagen type I, III and elastin is elevated in the PCLS model of IPF and can be restored back to vehicle levels using a phosphodiesterase inhibitor. Respir Res. 2016;17:76. doi: 10.1186/s12931-016-0394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brand OJ, Pasini A, Habgood A, Knox AJ, Jenkins G, Pang L. S52 suberanilohydroxamic acid (SAHA) inhibits collagen deposition in a transforming growth factor β1-driven precision cut lung slice (PCLS) model of pulmonary fibrosis. Thorax. 2016;71:A31.2–A31. [Google Scholar]
- 86.Barth K, Bläsche R, Kasper M. Lack of evidence for caveolin-1 and CD147 interaction before and after bleomycin-induced lung injury. Histochem Cell Biol. 2006;126:563–573. doi: 10.1007/s00418-006-0192-3. [DOI] [PubMed] [Google Scholar]
- 87.Kasper M, Seidel D, Knels L, Morishima N, Neisser A, Bramke S, et al. Early signs of lung fibrosis after in vitro treatment of rat lung slices with CdCl2 and TGF-beta1. Histochem Cell Biol. 2004;121:131–140. doi: 10.1007/s00418-003-0612-6. [DOI] [PubMed] [Google Scholar]
- 88.Obernolte H, Braunbach P, Jonigk D, Beinke S, Belyaev NN, Lennon M, et al. Transcriptomic analyses reveal anti-viral responses of epithelial cells and multiple immune cell types in HRV infected human lung tissue. Eur Respir J. 2017;50:PA4126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.