Abstract
Purpose
A subgroup of uveal melanoma (UM) gives rise to metastases at a late stage. Our objective was to identify patient and tumor characteristics that are associated with UM-related death in patients who survived 5 years following enucleation.
Methods
A retrospective analysis was performed in 583 primary UM cases, enucleated at the Leiden University Medical Center between 1983 and 2013. Univariable and multivariable Cox regression analyses were performed in the total cohort and separately in those surviving more than 5 years (n = 297).
Results
In the total cohort, the median age was 62.6 years, and the median tumor diameter was 12.0 mm. Monosomy 3 was detected in 53% of cases and gain of 8q in 47%. In the cohort surviving 5 years, the median age was 59.5 years, and the median tumor diameter was 11.0 mm. Monosomy 3 and gain of 8q were detected in 33% and 31% of cases, respectively. In the total cohort, male gender (P = 0.03), tumor diameter (P < 0.001), mitotic count (P < 0.001), extravascular matrix loops (P = 0.03), extraocular growth (P < 0.001), and gain of 8q (P < 0.001) were independently associated with UM-related death. In patients surviving 5 years after enucleation, univariable analysis revealed that age (P = 0.03), tumor diameter (P < 0.001), monosomy 3 (P = 0.04), and 8q gain (P = 0.003) were associated with subsequent UM-related death. Using a multivariable analysis, only male gender (P = 0.03) and gain of 8q (P = 0.01) remained significant.
Conclusions
Predictors of UM-related death change over time. Among UM patients who survived the initial 5 years following enucleation, male gender and chromosome 8q status were the remaining factors related to UM-related death later on.
Keywords: oncology, tumor, ocular, prognosis, genetics
Uveal melanoma (UM) is the most common type of ocular melanoma and originates from melanocytes residing in the uveal tract.1 It is the most frequently occurring primary intraocular malignancy in adults and is predominantly found in Caucasians, especially in those with fair skin and light iris color. The mean annual age-adjusted incidence is 5.1 per million in the United States.2 It is estimated that 6700 to 7100 new patients are diagnosed worldwide annually and that there are 87,000 to 106,000 survivors, many of whom are under surveillance for the development of overt metastases.3 UM metastasizes hematogenously, with a predilection for the liver. Up to 50% of patients die due to metastases within 10 years after diagnosis.4 The median survival time after the diagnosis of overt metastases ranges from 4 to 15 months.5,6
Clinical and histopathologic tumor characteristics such as largest basal diameter (LBD), thickness, ciliary body (CB) involvement, and extraocular growth (which together determine the American Joint Committee on Cancer [AJCC] Tumor, Node, Metastasis stage), as well as mitotic count, cell type, and extravascular matrix loops, have been related to the development of metastases.7–10 Additionally, genomic non-random chromosome alterations resulting in monosomy 3 and gain of 8q and a specific class 2 gene-expression profile have been associated with the occurrence of metastatic disease.11–13 Recently, mutations in specific genes such as BAP1, EIF1AX, and SF3B1 have been shown to have prognostic value in UM.14–17 Besides tumor characteristics, patient parameters such as gender and age may play a role in UM survival; males have been shown to have earlier and more frequent metastases in the first decade after diagnosis, whereas a lower rate of metastasis has been reported in younger patients.18–20
The moments when disseminated disease is detected and when it leads to death differ considerably among patients.21 Some UM patients (about 1.5%) have detectable metastases at the time of diagnosis of the primary tumor, whereas others develop clinical metastases more than 10 years after diagnosis.22,23
Although most studies report on parameters related to early death, little is known about how these parameters predict survival after an initial 5 years. Characteristics of the survivor group will likely differ from the original cohort at baseline, as those with unfavorable characteristics are—unfortunately—no longer included. Knowing the prognostic parameters for the surviving patients is important for patient counseling and may help researchers to understand the mechanisms that lead to late metastases.
We hypothesize that there will be a shift in prognostic parameters for patients who survived the initial 5 years. To study our hypothesis, we analyzed the association of patient characteristics and primary tumor features with UM-related survival in a cohort of 583 patients who had undergone primary enucleation for UM in our center.
Materials and Methods
Patients
Between March 1983 and December 2013, a total of 583 UM patients underwent primary enucleation at the Leiden Universiy Medical Center (LUMC). Baseline characteristics are shown in Table 1. Although we have kept a registry of enucleated UM patients since 1972, patients who underwent enucleation before 1983 (preceding the introduction of brachytherapy) were not included in the current study; they could have biased the results due to smaller tumor sizes, because at that time smaller tumors were also treated by enucleation. For this retrospective cohort analysis, we did not include patients who had undergone brachytherapy prior to enucleation, as the radiobiological effects of irradiation may influence UM histopathology and genetics.24–26
Table 1.
Characteristics at the Time of Enucleation of the Total Cohort of UM Patients Primarily Enucleated in the LUMC Between 1983 and 2013 (N = 583) and Patients Who Survived 5 Years Following Enucleation (n = 297)
| Characteristics | Total Cohort | 5-Year Survivors | P | |
|---|---|---|---|---|
| Gender, n (%) | 0.47 | |||
| Female | 272 (47) | 131 (44) | ||
| Male | 311 (53) | 166 (56) | ||
| Age at enucleation (y), median (range) | 62.6 (7.0–91.3) | 59.5 (7.0–88.3) | 0.001 | |
| Largest basal diameter (mm), median (range) | 12.0 (2.0–30.0) | 11.0 (2.0–24.0) | 0.003 | |
| Thickness (mm), median (range) | 6.0 (0.5–17.0) | 6.0 (0.5–17.0) | 0.253 | |
| Mitotic count, median (range) | 4 (0–35) | 4 (0–30) | 0.130 | |
| Ciliary body involvement, n (%) | 177 (30) | 62 (21) | 0.003 | |
| Cell type, n (%) | <0.001 | |||
| Spindle | 173 (30) | 125 (42) | ||
| Mixed/epithelioid | 408 (70) | 172 (58) | ||
| Extravascular matrix loops (determined in 378 and 176 cases), n (%) | 247 (65) | 102 (34) | 0.094 | |
| Extraocular growth, n (%) | 71 (12) | 22 (7) | 0.031 | |
| AJCC stage, n (%) | 0.001 | |||
| I | 112 (20) | 76 (26) | ||
| II | 342 (60) | 180 (61) | ||
| III | 119 (21) | 37 (13) | ||
| Monosomy 3 (determined in 275 and 113 cases), n (%) | 146 (53) | 37 (33) | <0.001 | |
| Gain of 8q (determined in 245 and 105 cases), n (%) | 115 (47) | 33 (31) | 0.007 | |
| Follow-up time (y), median (range) | 5.1 (0.08–33.3) | 10.5 (5.04–33.3) | <0.001 | |
| Metastases, n (%) | 235 (40) | 64 (22) | <0.001 | |
| Vital status, n (%) | 0.005 | |||
| Death due to UM metastases | 212 (36) | 52 (18) | ||
| Death due to other cause | 140 (24) | 75 (25) | ||
| Alive at last follow-up date | 231 (40) | 170 (57) | ||
Percentages, which are reported after exclusion of missing data, have been rounded and may not total 100.
The cause and date of death were obtained from the Integral Cancer Center West (a regional office of the Netherlands Comprehensive Cancer Organisation, https://iknl.nl/over-iknl/about-iknl). All Dutch cancer patients are reported to the Netherlands Comprehensive Cancer Organisation by their general physicians. Physicians employed by this institution are responsible for collecting information on the survival status of cancer patients by contacting the general physicians, who register the date and cause of death of their patients. We do not have information on the date of detection of overt metastases, as this was often not registered. Follow-up time, defined as the time period between primary enucleation and death or date of last follow-up, was updated in March 2017. The median follow-up time was 5.1 years (range, 0.08–33.3 years). At the last date of follow up, 212 patients had died due to UM metastases and 140 due to other causes, whereas 231 were alive (Table 1). Seven patients were lost to follow-up because of emigration. The Ethics Committee of the LUMC approved this study, which followed the tenets of the Declaration of Helsinki.
Histopathologic Examination
After enucleation, a portion of the tumor was snap frozen and stored at –80°C. The remaining tumor was fixed in 4% neutral-buffered formalin for 48 hours and embedded in paraffin. Hematoxylin and eosin-stained 4-µm-thick sections were analyzed by an ocular pathologist using a standard protocol for determination of histopathologic characteristics: LBD (mm), thickness (apical height, mm), mitotic count (per 2 mm2 at 40× magnification), tumor location, cell type (spindle, epithelioid, or mixed),27 and (since January 1991) the presence of extravascular matrix loops determined on PAS-stained slides without a counterstain28 and without a filter. Tumors were staged according to the 8th edition of the AJCC Cancer Staging Manual.29
Cytogenetic Analysis
Between 1999 and 2013, 291 UM samples were sent for cytogenetic analysis by karyotyping with or without FISH. In 62 of these and four other tumors, single-nucleotide polymorphism (SNP) analysis had been performed.30 GTG-banded (G-banding with Giemsa and trypsin) metaphases were used for karyotyping. Karyograms were analyzed using the automatic karyotyping software Cytovision (Leica Biosystems, Buffalo Grove, IL, USA) and described using the regulations of the International System for Human Cytogenetic Nomenclature (1995). Loss of a copy of chromosome 3 or gain of chromosome 8q in at least two cells was sufficient to classify the tumor as having the abnormality. If monosomy 3 was present in only one cell, the tumor was designated as a monosomy 3 tumor provided that other chromosome abnormalities common in UM were present. In case of 8q gain, the presence of an isochromosome 8q in only one cell was sufficient.
FISH was performed using DNA probes specific for the centromere of chromosome 3 (α-sat3 probe; Cytocell, Cambrigde, UK) and region 3p24.3–p25 (RP11-322M13 probe; Cytocell). Monosomy 3 or chromosome 8q status was assigned if the aberration was present in at least 10% of the analyzed cells. The Affymetrix GeneChip Mapping 250K Nsp assay and the Affymetrix CytoScan HD Array (Santa Clara, CA, USA) were used for SNP analyses. Copy numbers were determined using the Affymetrix Genotyping Console (GTC) and GTC Browser to visualize the data for analysis of the GeneChip Mapping 250K Nsp assay. The Affymetrix CytoScan HD arrays were analyzed using the Chromosome Analysis Suite. Different loci per chromosome were analyzed to adjust for partial gains or deletions. Approximately 200 probes per gene locus were averaged to determine copy numbers. In case of discrepancy between the tests, the tumor was classified as having monosomy 3 or chromosome 8q gain when either of the tests showed the abnormality.
Statistical Analysis
Data from clinical charts, pathology reports, and genetic tests and information on follow-up status were evaluated using SPSS Statistics 20 (IBM, Armonk, NY, USA). Five years was chosen as a cut-off value, as this follow-up period is commonly reported in epidemiologic studies in oncology. Characteristics between groups were compared using the Pearson's chi-square test for nominal categorical variables, the linear-by-linear association test for ordinal categorical variables, and the Mann–Whitney U test for continuous parameters.
Cox regression analyses were conducted in order to identify factors influencing survival. Effect estimates are reported as hazard ratios (HRs) with 95% confidence intervals (CIs) of death due to UM metastases by censoring for end of follow-up or death due to other causes. In addition to unadjusted univariable analyses, multivariable regression analyses were performed to evaluate independent effects. To evaluate the effect of deaths due to reasons other than UM metastases on the results of the regression analyses, competing risks regression analyses based on the Fine–Gray model were conducted, and cumulative incidence curves (package cmprsk) were generated and compared with the Gray's K-sample test using the statistical software package R (version 3.4.0).31–33 These analyses take other reasons of death into account as competing risks, which are treated as censored observations in conventional statistics such as Cox regression and Kaplan–Meier analyses. Censoring other causes of death exaggerates the apparent metastatic death rate.21 Regression analyses were performed in the total cohort of 583 patients as well as in those who survived, with or without metastases, more than 5 years after enucleation (n = 297). The competing risks regression analysis is the main regression model, whereas the Cox regression model is included for ease of comparison. All statistical tests were two sided, and P < 0.05 was considered to be statistically significant.
Results
Population Characteristics
Male patients comprised 53% of our total cohort of 583 UM patients. The median age at the time of enucleation was 62.6 years. The median tumor diameter was 12.0 mm, and the median tumor thickness was 6.0 mm. Almost one-third of cases showed ciliary body involvement. Whereas 20% of tumors were classified as AJCC stage I, most tumors (60%) were classified as AJCC stage II. Monosomy 3 was detected in 53% of the cases, and 47% harbored a gain of chromosome 8q. At last follow-up (median, 5.1 years), 235 patients (40%) were diagnosed with clinical UM metastases, and 212 patients (36%) had died due to UM metastases.
Five years after enucleation, 297 UM patients were alive (51%). Patient and tumor characteristics differed significantly between these patients and the original (n = 583) cohort. The median age at the time of enucleation was lower (59.5 years; P = 0.001), and tumors had a smaller median diameter (11.0 mm; P = 0.003) but not thickness (6.0 mm; P = 0.25). Ciliary body involvement was seen less often (21%; P = 0.003). Lower AJCC stages were seen more often, with 26% of tumors being AJCC stage I and 61% being stage II (P = 0.001). Monosomy 3 and gain of 8q were seen less frequently: in 33% of cases (P < 0.001) and in 31% of cases (P = 0.007), respectively. The complete overview of patient and tumor characteristics is provided in Table 1.
Outcome Analysis
In the patients who survived the first 5 years after enucleation for UM (n = 297), 64 patients (22%) had developed UM metastases and 52 patients (18%) had died of a UM-related cause at the last moment of follow-up (median, 10.5 years) (Table 1). We analyzed which prognostic factors were associated with UM-related death. The patients who died of a UM-related cause (compared to those who were alive at the end of follow-up or who died of another cause), demonstrated a larger LBD (P < 0.001) and higher mitotic count (P = 0.046), had a higher AJCC stage (P = 0.013), more often had monosomy 3 (P = 0.038), and had gain of chromosome 8q (P < 0.001) (Table 2).
Table 2.
Outcome Analysis of Those Who Survived 5 Years Following Enucleation (N = 297)
| Characteristics | Alive/Death Due to Other Causes (n = 245) | UM-Related Death (n = 52) | P | |
|---|---|---|---|---|
| Gender, n (%) | 0.53 | |||
| Female | 106 (43) | 25 (48) | ||
| Male | 139 (57) | 27 (52) | ||
| Age at enucleation (y), median (range) | 59.4 (7.0–88.3) | 60.4 (28.7–87.7) | 0.57 | |
| Largest basal diameter (mm), median (range) | 11.0 (2.0–20.0) | 12.0 (3.0–24.0) | 0.001 | |
| Thickness (mm), median (range) | 6.0 (0.5–17.0) | 7.0 (0.8–15.0) | 0.17 | |
| Mitotic count, median (range) | 4 (0–25) | 5 (0–30) | 0.046 | |
| Ciliary body involvement, n (%) | 50 (20) | 12 (23) | 0.67 | |
| Cell type, n (%) | 0.97 | |||
| Spindle | 103 (42) | 22 (42) | ||
| Mixed/epithelioid | 142 (58) | 30 (58) | ||
| Extravascular matrix loops (determined in 176 cases), n (%) | 84 (56) | 18 (72) | 0.13 | |
| Extraocular growth, n (%) | 16 (7) | 6 (12) | 0.22 | |
| AJCC stage, n (%) | 0.013 | |||
| I | 66 (27) | 10 (19) | ||
| II | 151 (63) | 29 (56) | ||
| III | 24 (10) | 13 (25) | ||
| Monosomy 3 (determined in 113 cases), n (%) | 29 (29) | 8 (57) | 0.038 | |
| Gain of 8q (determined in 105 cases), n (%) | 24 (26) | 9 (82) | <0.001 | |
| Follow-up time (y), median (range) | 12.1 (5.09–33.3) | 7.3 (5.04–21.53) | <0.001 | |
| Metastases, n (%) | 12 (5) | 52 (100) | <0.001 | |
| Vital status | <0.001 | |||
| Death due to UM metastases | 0 (0) | 52 (100) | ||
| Death due to other cause | 75 (31) | 0 (0) | ||
| Alive at last follow-up date | 170 (69) | 0 (0) | ||
Percentages, which are reported after exclusion of missing data, have been rounded and may not total 100.
Regression Analyses
To study the univariable and multivariable effect of all prognostic parameters on patient survival, we conducted Cox regression and competing risks regression analyses (Table 3). In the total cohort, the multivariable competing risks regression model showed male gender (hazard ratio [HR], 1.91; 95% CI, 1.08–3.36; P = 0.03), increasing tumor diameter (HR, 1.16; 95% CI, 1.08–1.24; P < 0.001), and mitotic count (HR, 1.09; 95% CI, 1.05–1.13; P < 0.001), the presence of extravascular matrix loops (HR, 2.28; 95% CI: 1.08–4.8; P = 0.03), extraocular growth (HR, 2.73; 95% CI, 1.59–4.7; P < 0.001), and gain of 8q (HR, 3.97; 95% CI, 1.96–8.04; P < 0.001) to be significantly associated with UM-related death (Table 3A). Except for age at enucleation and extravascular matrix loops (P = 0.05), which were associated with the risk of death due to UM metastases in the multivariable Cox regression model, the results of both models were in concordance.
Table 3.
Cox Regression and Competing Risk Regression Analyses with Death Due to UM Metastases as the Endpoint of Interest, Evaluating the Effect of Patient and Tumor Characteristics on Survival
| Univariable Cox Regression | Multivariable Cox Regression | Multivariable Competing Risks Regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P |
| A: Total Cohort (N = 583) | |||||||||
| Male gender | 0.84 | 0.64–1.1 | 0.21 | 2.19 | 1.25–3.86 | 0.006 | 1.91 | 1.08–3.36 | 0.03 |
| Age at enucleation | 1.03 | 1.02–1.04 | <0.001 | 1.03 | 1.01–1.05 | 0.01 | 1.02 | 0.99–1.04 | 0.12 |
| Largest basal diameter | 1.17 | 1.13–1.21 | <0.001 | 1.15 | 1.06–1.24 | <0.001 | 1.16 | 1.08–1.24 | <0.001 |
| Thickness | 1.08 | 1.03–1.13 | <0.001 | 0.93 | 0.84–1.02 | 0.1 | 0.93 | 0.84–1.03 | 0.17 |
| Mitotic count | 1.06 | 1.04–1.08 | <0.001 | 1.09 | 1.04–1.13 | <0.001 | 1.09 | 1.05–1.13 | <0.001 |
| Ciliary body involvement | 2.13 | 1.61–2.81 | <0.001 | 1.35 | 0.8–2.26 | 0.26 | 1.2 | 0.72–2.0 | 0.49 |
| Mixed/epithelioid cell type | 2.56 | 1.81–3.62 | <0.001 | 1.29 | 0.62–2.69 | 0.5 | 1.3 | 0.67– 2.54 | 0.44 |
| Extravascular matrix loops (known in 378 cases) | 2.95 | 1.89–4.63 | <0.001 | 2.13 | 0.99–4.55 | 0.05 | 2.28 | 1.08–4.8 | 0.03 |
| Extraocular growth | 2.17 | 1.53–3.07 | <0.001 | 2.76 | 1.54–4.94 | 0.001 | 2.73 | 1.59–4.7 | <0.001 |
| Monosomy 3 (known in 275 cases) | 5.42 | 3.26–9.01 | <0.001 | 1.74 | 0.8–3.77 | 0.16 | 1.51 | 0.72–3.13 | 0.27 |
| Gain of 8q (known in 245 cases) | 5.92 | 3.5–10.02 | <0.001 | 4.67 | 2.24–9.75 | <0.001 | 3.92 | 1.96–7.84 | <0.001 |
| B: After 5-Year Follow-Up (n = 297) | |||||||||
| Male gender | 0.99 | 0.57–1.69 | 0.94 | 6.21 | 0.56–69.04 | 0.14 | 6.87 | 1.26–37.42 | 0.03 |
| Age at enucleation | 1.03 | 1.0–1.05 | 0.03 | 1.04 | 0.96–1.11 | 0.34 | 1.03 | 0.95–1.11 | 0.46 |
| Largest basal diameter | 1.14 | 1.07–1.22 | <0.001 | 1.17 | 0.85–1.6 | 0.34 | 1.11 | 0.82–1.51 | 0.49 |
| Thickness | 1.09 | 1.0–1.18 | 0.05 | 0.84 | 0.6–1.19 | 0.33 | 0.87 | 0.52–1.47 | 0.61 |
| Mitotic count | 1.02 | 0.97–1.07 | 0.42 | 1.23 | 0.95–1.58 | 0.12 | 1.24 | 0.88–1.74 | 0.21 |
| Ciliary body involvement | 1.47 | 0.77–2.82 | 0.24 | 1.58 | 0.18–13.67 | 0.68 | 1.65 | 0.06–42.67 | 0.76 |
| Mixed/epithelioid cell type | 1.12 | 0.65–1.95 | 0.68 | 0.42 | 0.04–4.11 | 0.46 | 0.47 | 0.04–5.86 | 0.56 |
| Extravascular matrix loops (known in 176 cases) | 2.06 | 0.86–4.95 | 0.11 | 5.37 | 0.32–89.78 | 0.24 | 6.1 | 0.55–67.53 | 0.14 |
| Extraocular growth | 1.58 | 0.67–3.69 | 0.29 | 5.24 | 0.11–246.85 | 0.4 | 6.96 | 0.08–593.39 | 0.39 |
| Monosomy 3 (known in 113 cases) | 3.12 | 1.08–9.02 | 0.04 | 1.82 | 0.21–15.68 | 0.59 | 1.87 | 0.29–12.28 | 0.51 |
| Gain of 8q (known in 105 cases) | 10.31 | 2.23–47.74 | 0.003 | 15.1 | 1.69–135.07 | 0.02 | 14.75 | 1.77–122.99 | 0.01 |
Significant P values are in bold.
We subsequently separately analyzed those who were still alive at 5 years after enucleation for UM. Univariable Cox regression demonstrated that in this group, age at enucleation (HR, 1.03; 95% CI, 1.0–1.05; P = 0.03), largest basal diameter (HR, 1.14; 95% CI, 1.07–1.22; P < 0.001), monosomy 3 (HR, 3.12; 95% CI, 1.08–9.04; P = 0.04), and gain of 8q (HR, 10.31; 95% CI, 2.23–47.74; P = 0.003) were related to UM-related death. After correction in a multivariable competing risk regression, only male gender (HR, 6.87; 95% CI, 1.26–37.42; P = 0.03) and gain of 8q (HR, 14.75; 95% CI, 1.77–122.99; P = 0.01) were independently associated with UM-related death (Table 3B). To visualize the effect of gender and chromosome 8q status on patient survival 5 years following enucleation, we generated cumulative incidence curves that took competing risks into account (Fig.). Cumulative incidence curves did not show a significant difference in incidence of UM-related death between men and women (P = 0.78) (Fig. A), whereas gain of 8q was associated with poor survival throughout the whole follow-up period (P < 0.001) (Fig. B).
Figure.
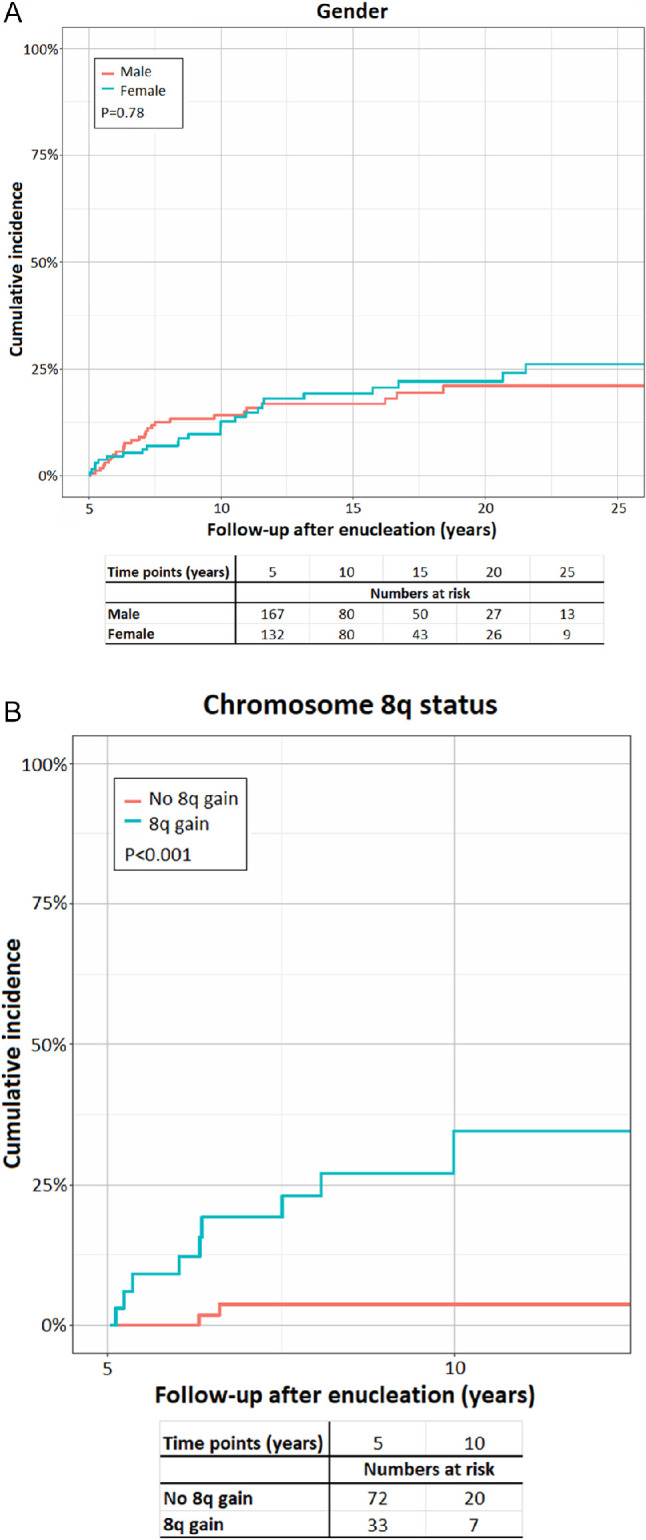
Cumulative incidence curves depicting the effect of gender (A) and chromosome 8q status (B) on the incidence of UM-related death 5 years after enucleation.
Discussion
This study shows that patient and tumor characteristics of UM patients who have survived more than 5 years differ from those who died earlier, as expected. Although various prognostic factors have been identified for patients at the moment of diagnosis, we observed that a higher age, larger LBD, loss of chromosome 3, and gain of chromosome 8q are associated with adverse prognosis once patients survive 5 years following enucleation. Using a multivariable competing risk analysis, only male gender and gain of chromosome 8q remained independently associated with adverse prognosis.
Several previous studies have reported on patient and tumor characteristics at the moment of diagnosis of long-term metastasized UM survivors and factors influencing long-term prognosis of UM patients.21–23 All of these studies started at the moment of primary tumor treatment. Rietschel et al.22 found that female gender and younger age at diagnosis of metastatic melanoma were predictive of a prolonged survival in patients with metastatic UM, and a later study by Buzacco et al.23 corroborated the association of age. Comparable results were reported in a large study by Shields et al.,19 in which older patients were found to die of metastases significantly more often than their younger counterparts, and by Damato et al.,20 who reported larger tumors with a higher malignancy degree in older patients. A large population-based survey by Bergman et al.34 in Swedish UM patients identified younger age as being associated with better survival. However, Kujala et al.,21 who performed multivariate competing risks regression analyses (taking competing risks of death into account), found no influence of age and a borderline effect of gender on survival in a cohort of 289 primary UM with long-term follow-up. In accordance with the study by Kujala et al., our competing risks regression analysis showed no effect of age at enucleation on survival and demonstrated that gender independently influences survival in UM patients (Table 3A).
Male gender has been associated with increased risk of death from all causes and with worse survival after metastases have developed.22,35 A study by Zloto et al.18 showed that male gender predicted earlier and more frequent metastases in the first decade after diagnosis of the primary tumor and also found gender to be important for the rate at which metastases appear and their apparent aggressiveness. A recent study in 344 South Korean UM patients reported higher survival probabilities in females.36
Several explanations can be proposed for the observed association of male gender with poor survival in our study, which was evident in the total cohort, as well as after 5 years of follow-up, in our competing risks regression analysis. One possibility is that males have larger and more advanced primary tumors than females, as described in some but not all reports.18,37 The larger tumor size in men could be due to tumors of a higher malignancy grade or a delay in diagnosis and treatment (lead-time bias) in males.18,38 However, male gender was independently associated with poor survival by multivariable competing risks regression analysis. A more likely explanation for worse survival of males could be their potentially poorer general health, making them more vulnerable than women to metastases of comparable malignancy grades. A third explanation could be a contributing effect of testosterone on the growth of micrometastases or the effects of a less efficient immune surveillance on the outgrowth of metastases in men.18 This might be related to the differential way sex hormones interact with the immune system. Likewise, males and females are known to differ, for example, in their risk of autoimmune diseases.39 However, the exact role of gonadal hormones in UM survival remains unclear. Studies that have explored the potential role of female hormones in the etiology of UM and the risk of metastases have found no evidence of such a relationship.40–42
Older age at enucleation was associated with early UM-related death and influenced survival in the total cohort according to the Cox regression analysis. However, the competing risks regression analysis did not identify age as an independent factor influencing survival, suggesting that the independent association of older age and worse survival in our Cox regression analysis and in earlier studies is based on biases induced by ignoring competing risks as proposed earlier by Kujala et al.21
Anatomic and histologic tumor features (large tumor diameter, high mitotic count, extraocular growth, and the presence of extravascular matrix loops) were independently associated with poor survival in the total cohort. Remarkably, none of the anatomic and histologic tumor parameters still influenced survival after 5 years of follow-up. Primary UMs possessing these characteristics may give rise to more aggressive metastases that cause death early on, after which a more homogeneous group of patients remains in which survival probability is more strongly determined by (the combination of) other factors, such as gender and chromosome status.
Gain of 8q was the parameter with the highest HR that showed an independent association with poor survival in the total cohort as well as specifically 5 years following treatment of the primary tumor. As expected, tumors with more than 5 years of follow-up demonstrated more often disomy 3 (67%) compared to the total cohort (47%). Other studies have shown that late-death cases may show disomy 3/gene-expression profile class 1 with overexpression of PRAME or SF3B1 mutation.15,43,44 Recently, PRAME overexpression has been shown to be associated with metastatic risk in class 1 tumors, and the SF3B1 mutation is similarly associated with the development of metastases in disomy 3 tumors.43,44 Interestingly, in a recent study by The Cancer Genome Atlas, SF3B1 mutations in disomy 3 tumors were found to occur in combination with (partial) 8q gain.45 As we do not know the mutation status of our tumors, we cannot evaluate survival in relation to different types of mutations.44
Remarkably, in a multivariable regression analysis, chromosome 3 status was not independently associated with prognosis throughout the entire follow-up period nor after 5 years of follow-up. We hypothesize that the effect of monosomy 3, which has been shown to be strongly correlated to histopathologic tumor features, on survival was decreased by the presence of anatomic and histologic tumor features that we have evaluated in our model. This may be especially true for extravascular matrix loops, which have been shown to correlate strongly with monosomy 3 and may even be used to predict monosomy 3 accurately in 70% of cases when considered together with cell type.46,47 In accordance with this, we did find an independent effect of chromosome 3 status on survival in the total cohort if we excluded extravascular matrix loops from our regression model (data not shown).
The major strength of our study is the availability of long-term follow-up data, which was achieved due to the organization of health care in the Netherlands. We were able to identify a cohort of 297 patients who survived 5 years after enucleation and could investigate the prognostic factors within this group. With our competing risk regression analysis, we believe that we applied a robust methodology. As the causes of death, other than UM-related, may vary between countries, our results must be confirmed in other populations.
A limitation of our study is that it was restricted to patients who underwent primary enucleation, which makes our results not applicable to patients undergoing eye-preserving treatment options. Another limitation is the fact that chromosome 3 and 8q status was not known in approximately half of the patients and was determined by karyotyping and FISH in most of the cases. We believe that the true percentage of tumors having monosomy 3 or gain of 8q may be higher when determined by newer and more sensitive techniques such as SNP-based copy number determination. We began performing SNP-based analyses in our clinic in September 2015. Of unknown importance is the lead-time bias, as patients with larger primary tumors seemed to have a shorter survival because the tumors were detected later on.48
Conclusions
Our study shows that the subgroup of patients who survive UM for at least 5 years differs from those who die early and that prognostic factors at 5 years after enucleation differ from those at the time of enucleation. At the 5-year point, male gender and gain of 8q are the only independent predictors of poor outcome later on.
Acknowledgments
Supported by grants from the Eye Cancer Foundation (New York, NY, USA), Stichting Blindenpenning (the Netherlands), Horizon 2020 grant no. 667787 UM CURE (European Commission), and the Sigrid Juselius Foundation (Finland). The authors alone are responsible for the content and writing of the paper.
Disclosure: M. Dogrusöz, None; N.J. Brouwer, None; S.J.R. de Geus, None; L.V. Ly, None; S. Böhringer, None; S.G. van Duinen, None; W.G.M. Kroes, None; P.A. van der Velden, None; G.W. Haasnoot, None; M. Marinkovic, None; G.P.M. Luyten, None; T.T. Kivelä, None; M.J. Jager, None
References
- 1. Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998; 83: 1664–1678. [DOI] [PubMed] [Google Scholar]
- 2. Mahendraraj K, Shrestha S, Lau CS, Chamberlain RS. Ocular melanoma-when you have seen one, you have not seen them all: a clinical outcome study from the Surveillance, Epidemiology and End Results (SEER) database (1973-2012). Clin Ophthalmol. 2017; 11: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kivelä T. Incidence, prevalence and epidemiology of ocular melanoma. In: Murray T, Boldt HC, eds. Ocular Melanoma: Advances in Diagnostic and Therapeutic Strategies. London: Future Medicine Ltd; 2014: 20–38. [Google Scholar]
- 4. Gamel JW, McLean IW, McCurdy JB. Biologic distinctions between cure and time to death in 2892 patients with intraocular melanoma. Cancer. 1993; 71: 2299–2305. [DOI] [PubMed] [Google Scholar]
- 5. Singh AD, Borden EC. Metastatic uveal melanoma. Ophthalmol Clin North Am. 2005; 18: 143–150, ix. [DOI] [PubMed] [Google Scholar]
- 6. Augsburger JJ, Correa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol. 2009; 148: 119–127. [DOI] [PubMed] [Google Scholar]
- 7. Kujala E, Damato B, Coupland SE, et al.. Staging of ciliary body and choroidal melanomas based on anatomic extent. J Clin Oncol. 2013; 31: 2825–2831. [DOI] [PubMed] [Google Scholar]
- 8. McLean MJ, Foster WD, Zimmerman LE. Prognostic factors in small malignant melanomas of choroid and ciliary body. Arch Ophthalmol. 1977; 95: 48–58. [DOI] [PubMed] [Google Scholar]
- 9. McLean IW, Foster WD, Zimmerman LE, Gamel JW. Modifications of Callender's classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol. 1983; 96: 502–509. [DOI] [PubMed] [Google Scholar]
- 10. Folberg R, Pe'er J, Gruman LM, et al.. The morphologic characteristics of tumor blood vessels as a marker of tumor progression in primary human uveal melanoma: a matched case-control study. Hum Pathol. 1992; 23: 1298–1305. [DOI] [PubMed] [Google Scholar]
- 11. Prescher G, Bornfeld N, Hirche H, Horsthemke B, Jockel KH, Becher R. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996; 347: 1222–1225. [DOI] [PubMed] [Google Scholar]
- 12. Sisley K, Rennie IG, Parsons MA, et al.. Abnormalities of chromosomes 3 and 8 in posterior uveal melanoma correlate with prognosis. Genes Chromosomes Cancer. 1997; 19: 22–28. [DOI] [PubMed] [Google Scholar]
- 13. Onken MD, Worley LA, Tuscan MD, Harbour JW. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn. 2010; 12: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harbour JW, Onken MD, Roberson ED, et al.. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010; 330: 1410–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin M, Masshofer L, Temming P, et al.. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat Genet. 2013; 45: 933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Furney SJ, Pedersen M, Gentien D, et al.. SF3B1 mutations are associated with alternative splicing in uveal melanoma. Cancer Discov. 2013; 3: 1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van de Nes JA, Nelles J, Kreis S, et al.. Comparing the prognostic value of BAP1 mutation pattern, chromosome 3 status, and BAP1 immunohistochemistry in uveal melanoma. Am J Surg Pathol. 2016; 40: 796–805. [DOI] [PubMed] [Google Scholar]
- 18. Zloto O, Pe'er J, Frenkel S. Gender differences in clinical presentation and prognosis of uveal melanoma. Invest Ophthalmol Vis Sci. 2013; 54: 652–656. [DOI] [PubMed] [Google Scholar]
- 19. Shields CL, Kaliki S, Furuta M, Mashayekhi A, Shields JA. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina. 2012; 32: 1363–1372. [DOI] [PubMed] [Google Scholar]
- 20. Damato BE, Heimann H, Kalirai H, Coupland SE. Age, survival predictors, and metastatic death in patients with choroidal melanoma: tentative evidence of a therapeutic effect on survival. JAMA Ophthalmol. 2014; 132: 605–613. [DOI] [PubMed] [Google Scholar]
- 21. Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003; 44: 4651–4659. [DOI] [PubMed] [Google Scholar]
- 22. Rietschel P, Panageas KS, Hanlon C, Patel A, Abramson DH, Chapman PB. Variates of survival in metastatic uveal melanoma. J Clin Oncol. 2005; 23: 8076–8080. [DOI] [PubMed] [Google Scholar]
- 23. Buzzacco DM, Abdel-Rahman MH, Park S, Davidorf F, Olencki T, Cebulla CM. Long-term survivors with metastatic uveal melanoma. Open Ophthalmol J. 2012; 6: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Avery RB, Diener-West M, Reynolds SM, Grossniklaus HE, Green WR, Albert DM. Histopathologic characteristics of choroidal melanoma in eyes enucleated after iodine 125 brachytherapy in the collaborative ocular melanoma study. Arch Ophthalmol. 2008; 126: 207–212. [DOI] [PubMed] [Google Scholar]
- 25. Toivonen P, Makitie T, Kujala E, Kivela T. Macrophages and microcirculation in regressed and partially regressed irradiated choroidal and ciliary body melanomas. Curr Eye Res. 2003; 27: 237–245. [DOI] [PubMed] [Google Scholar]
- 26. Dogrusoz M, Kroes WG, van Duinen SG, et al.. Radiation treatment affects chromosome testing in uveal melanoma. Invest Ophthalmol Vis Sci. 2015; 56: 5956–5964. [DOI] [PubMed] [Google Scholar]
- 27. Font RL, Croxatto O, Rao NA. AFIP Atlas of Tumor Pathology: Tumors of the Eye and Ocular Adnexa. Silver Spring, MD: American Registry of Pathology; 2006: 56–60. [Google Scholar]
- 28. Folberg R, Rummelt V, Parys-Van Ginderdeuren R, et al.. The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology. 1993; 100: 1389–1398. [DOI] [PubMed] [Google Scholar]
- 29. Kivela T, Simpson ER, Grossniklaus HE, et al.. Uveal melanoma. In: Amin MB Edge SB Green FL, et al., eds. AJCC Cancer Staging Manual. 8th ed New York, NY: Springer; 2017: 805–817. [Google Scholar]
- 30. Dogrusoz M, Bagger M, van Duinen SG, et al.. The prognostic value of AJCC staging in uveal melanoma is enhanced by adding chromosome 3 and 8q status. Invest Ophthalmol Vis Sci. 2017; 58: 833–842. [DOI] [PubMed] [Google Scholar]
- 31. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988; 16: 1141–1154. [Google Scholar]
- 32. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999; 94: 496–509. [Google Scholar]
- 33. R Development Core Team. R: a language and environment for statistical computing. Available at: http://www.r-project.org/. Accessed July 5, 2017.
- 34. Bergman L, Seregard S, Nilsson B, Lundell G, Ringborg U, Ragnarsson-Olding B. Uveal melanoma survival in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci. 2003; 44: 3282–3287. [DOI] [PubMed] [Google Scholar]
- 35. Isager P, Ehlers N, Overgaard J. Prognostic factors for survival after enucleation for choroidal and ciliary body melanomas. Acta Ophthalmol Scand. 2004; 82: 517–525. [DOI] [PubMed] [Google Scholar]
- 36. Park SJ, Oh CM, Yeon B, Cho H, Park KH. Sex disparity in survival of patients with uveal melanoma: better survival rates in women than in men in South Korea. Invest Ophthalmol Vis Sci. 2017; 58: 1909–1915. [DOI] [PubMed] [Google Scholar]
- 37. Damato BE, Coupland SE. Differences in uveal melanomas between men and women from the British Isles. Eye (Lond). 2012; 26: 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Virgili G, Gatta G, Ciccolallo L, et al.. Survival in patients with uveal melanoma in Europe. Arch Ophthalmol. 2008; 126: 1413–1418. [DOI] [PubMed] [Google Scholar]
- 39. Voskuhl R. Sex differences in autoimmune diseases. Biol Sex Differ. 2011; 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Behrens T, Kaerlev L, Cree I, et al.. Hormonal exposures and the risk of uveal melanoma. Cancer Causes Control. 2010; 21: 1625–1634. [DOI] [PubMed] [Google Scholar]
- 41. Holly EA, Aston DA, Ahn DK, Kristiansen JJ, Char DH. Uveal melanoma, hormonal and reproductive factors in women. Cancer Res. 1991; 51: 1370–1372. [PubMed] [Google Scholar]
- 42. Egan KM, Walsh SM, Seddon JM, Gragoudas ES. An evaluation of the influence of reproductive factors on the risk of metastases from uveal melanoma. Ophthalmology. 1993; 100: 1160–1165; discussion 1166. [DOI] [PubMed] [Google Scholar]
- 43. Field MG, Durante MA, Decatur CL, et al.. Epigenetic reprogramming and aberrant expression of PRAME are associated with increased metastatic risk in Class 1 and Class 2 uveal melanomas. Oncotarget. 2016; 7: 59209–59219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yavuzyigitoglu S, Koopmans AE, Verdijk RM, et al.. Uveal melanomas with SF3B1 mutations: a distinct subclass associated with late-onset metastases. Ophthalmology. 2016; 123: 1118–1128. [DOI] [PubMed] [Google Scholar]
- 45. Robertson AG, Shih J, Yau C, et al.. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell. 2018; 33: 151. [DOI] [PubMed] [Google Scholar]
- 46. Scholes AG, Damato BE, Nunn J, Hiscott P, Grierson I, Field JK. Monosomy 3 in uveal melanoma: correlation with clinical and histologic predictors of survival. Invest Ophthalmol Vis Sci. 2003; 44: 1008–1011. [DOI] [PubMed] [Google Scholar]
- 47. Sandinha MT, Farquharson MA, McKay IC, Roberts F. Monosomy 3 predicts death but not time until death in choroidal melanoma. Invest Ophthalmol Vis Sci. 2005; 46: 3497–3501. [DOI] [PubMed] [Google Scholar]
- 48. Damato B, Eleuteri A, Taktak AF, Coupland SE. Estimating prognosis for survival after treatment of choroidal melanoma. Prog Retin Eye Res. 2011; 30: 285–295. [DOI] [PubMed] [Google Scholar]


