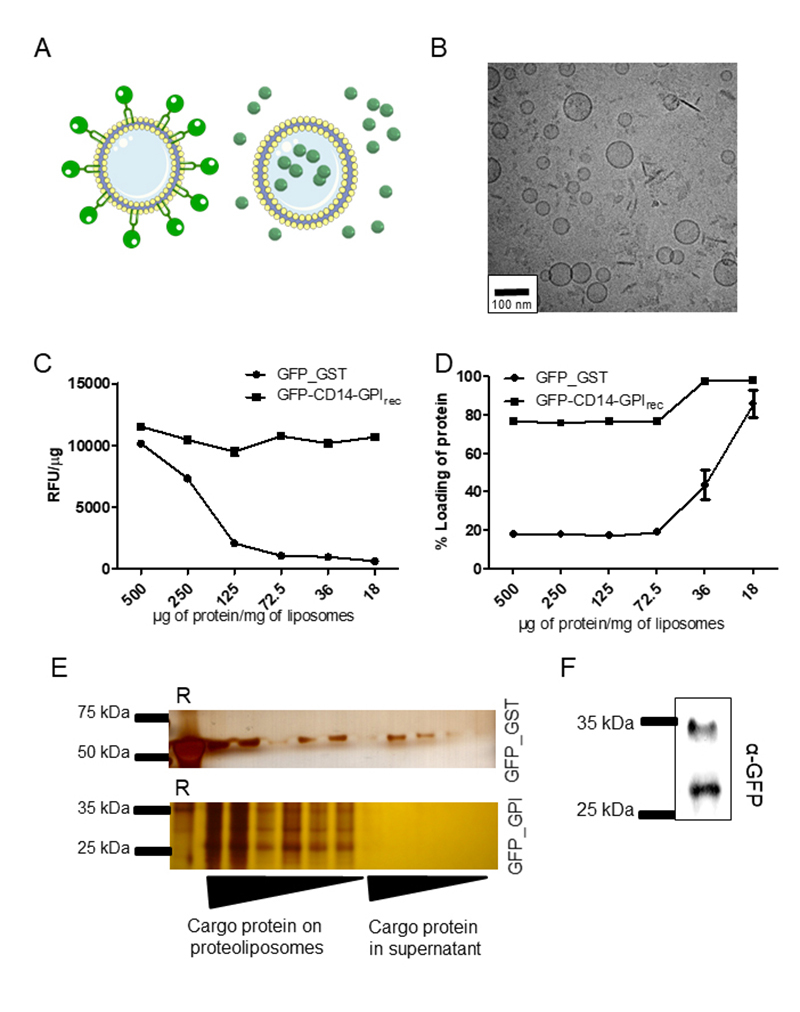
Figure 2. Incorporation of GFP-CD14 onto liposomes by its lipophilic domain is superior to incorporation of GFP in liposomes by simple co-incubation. (A) Proposed model of liposomes with either GFP fused to CD14-GPIrec localizing outside of liposomes (left side) or soluble GST-GFP (right side). (B) Cryo-TEM fracture images of proteoliposomes formed after incubation with GFP-CD14-GPIrec (72.5 µg of protein/mg lipid). (C) To show dose-dependent incorporation of recombinant proteins, the relative fluorescence in proteoliposomes emitted by GFP (Relative fluorescence units, RFU) was analyzed by flow cytometry and normalized to the amount of protein used to form proteoliposomes (RFU/µg of protein). The amount of protein used to form proteoliposomes was varied (ranging from 500 µg to 18 µg) while maintaining a fixed quantity of 1 mg of total lipids to form liposomes. This experiment was done twice in triplicate. (D) Formed proteoliposomes were ultra-centrifuged and the amount of protein incorporated in each batch was measured in proteoliposomes (pellet) and in the supernatant fraction. The amount of protein retained in proteoliposomes from incubation of GFP-GST (soluble form of GFP) or GFP-CD14-GPIrec (membrane-attached form of GFP extracted from transfected CHO cells) is expressed as a percentage of protein loaded in proteoliposomes. This experiment was also done twice in triplicate. (E) To demonstrate a dose-dependent incorporation of recombinant proteins/proteoliposomes obtained after ultracentrifugation, aliquots from pellets of proteoliposomes and supernatants were submitted to SDS-PAGE electrophoresis followed by silver staining. The complete pellet fraction of formed proteoliposomes and 20 µL of the supernatant of each batch were applied in a sequence. The first lane of each gel is the recombinant protein used in each experiment alone (“R”). (F) GFP on proteoliposomes can be detected by anti-GFP antibodies. The proteoliposome used was obtained from a batch loaded with 72.5 µg GFP-CD14-GPIrec per mg lipid after ultracentrifugation. Note that GFP is partly uncoupled from the CD14-GPI peptide leading to full-length (upper band) and GFP-only protein species (lower band). Empty liposomes receiving the same treatment showed no signal with the anti-GFP used herein (data not shown).