Arora and Olkkonen discuss a recent study from Pedersen et al. on the role of Protrudin in cancer cell invasion.
Abstract
Invadopodia are dynamic protrusions that harbor matrix metalloproteinases for pericellular matrix degradation. However, the mechanisms underlying their maturation are poorly understood. Pedersen et al. (2020. J. Cell Biol. https://doi.org/10.1083/jcb.202003063) demonstrate a dual role of Protrudin in invadopodia elongation and matrix degradation, central to cell invasion and cancer metastasis.
Invadopodia are invasive cell surface protrusions that play a central role in tumor metastasis. These actin-rich structures harbor metalloproteinases that upon exocytosis degrade the surrounding extracellular matrix (ECM; 1). This pathological proteolytic activity offers a spearheaded force for the growing tumor cells to the gain access to the bloodstream and spread to distant tissues. Invadopodia facilitate the invasion of different substrates/environments by the cancerous cells and thus contribute at each step of metastasis. This includes degradation of local basement membrane, invasion of the ECM, intravasation to enter the bloodstream and extravasation to metastasize at distant tissue sites (2). It is therefore crucial to understand the mechanistic intricacies involved in the formation and function of these protrusive structures.
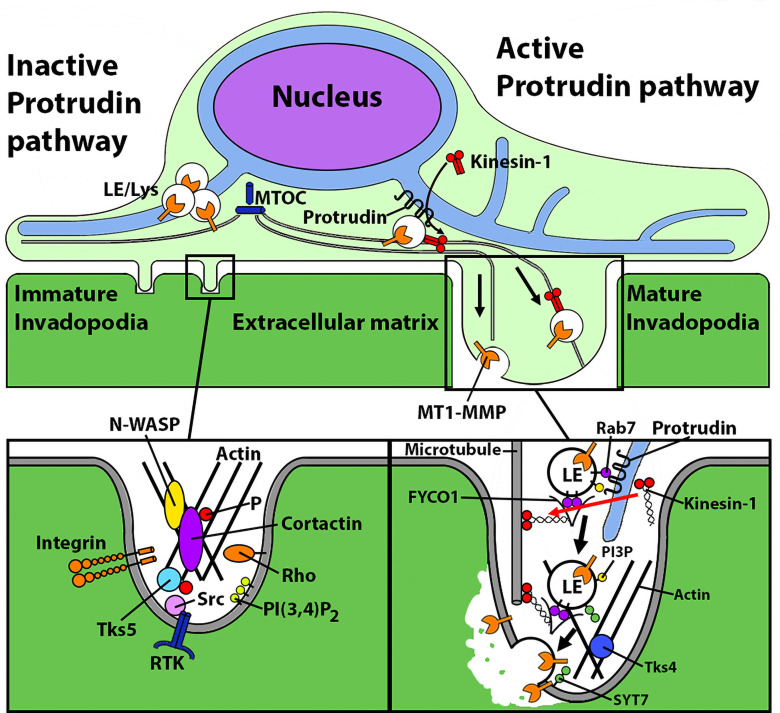
Growth factor stimulation or signals from ECM initiate the formation of invadopodia, wherein a dense core of filamentous actin and cortactin is surrounded by adaptor proteins, scaffold proteins, and signaling molecules such as integrins, kinases, and Rho GTPases (Fig. 1). Activation of Src and PKC signaling pathways and phosphorylation of cortactin are the key events for actin polymerization and the invadopodia precursor assembly step (3, 4). This is accompanied by enhanced production of phosphatidylinositol-3,4-bisphosphate (PI(3,4)P2) at the plasma membrane (PM) close to the site of precursor formation. Tks5, a scaffold protein and substrate of Src, binds to PI(3,4)P2 at the PM and plays a key role in stabilization of the precursor complex by anchoring it to the PM. Once stabilized, the complex elongates to form directional protrusions containing MMPs that upon exocytosis degrade the pericellular ECM, thus promoting invadopodia maturation (5).
Figure 1.
Protrudin plays a dual role in invadopodia maturation. Schematic of Protrudin function as demonstrated in the study by Pedersen et al. (7). Left: Initiation of invadopodia formation, independent of Protrudin. Right: Invadopodia elongation and maturation, which require the Protrudin pathway. Protrudin promotes ER–endosome membrane contacts at which kinesin-1 is loaded onto FYCO1 on MT1-MMP-laden late endosomes (LE) to drive their anterograde transport and exocytosis, thus facilitating (a) invadopodia membrane extension and (b) ECM degradation/invasion. MTOC, microtubule-organizing center; P, phosphate; Tks5, tyrosine kinase substrate with 5 SH3 domains; RTK, receptor tyrosine kinase; SYT7, synaptotagmin 7.
MMPs are expressed by nearly all invasive cancers and are linked to tumor aggressiveness and prognosis. They are established as promising therapeutic targets. Of these, MT1-MMP accumulates at the invadopodia of tumor cells and is one of the key enzymes in tumor invasion. It is an endosomal transmembrane protease which is delivered to the growing invadopodia via vesicular trafficking. Extensive studies have been undertaken to understand the regulation of its endocytic uptake, trafficking within the cells, and PM delivery (6).
The study by Pedersen et al. (7) in this issue provides new mechanistic insights into invadopodia maturation. The results demonstrate a key role of Protrudin, a protein of endoplasmic reticulum (ER)-endosome membrane contact sites, in invadopodia elongation and MT1-MMP exocytosis for matrix degradation by cancerous cells. Protrudin is highly expressed in brain, especially cerebrum and cerebellum, and is important for neurite outgrowth (8). Besides brain, it is also expressed in many nonneuronal tissues; however, its role therein is largely unknown. Protrudin is an ER transmembrane protein that contains a Rab11-binding domain, a low complexity region (LCR) that binds Rab7 on late endosomes (LE), and a FYVE domain that binds endosomal phosphatidylinositol 3-phosphate (PI3P). Previous studies have dissected the role of each domain of this protein to elucidate its function in maintaining ER morphology and neurite growth (8, 9). In an earlier study, the present authors demonstrated that Protrudin promotes directional translocation of endosomes toward cell periphery to provide membrane for neurite outgrowth (10). Repeated interactions of Protrudin at the ER and endosomes are necessary for loading kinesin-1 motors onto the FYCO1 adaptor protein of LE. The motor protein then drives anterograde movement of the LE and facilitates their synaptotagmin VII (SYT7)-dependent fusion with the PM.
Pedersen et al. propose a similar role of Protrudin in MDA-MB-231 cells, an invasive breast cancer cell line. Protrudin interacts with MT-MMP1-laden, Rab7-positive LE and promotes FYCO1-mediated loading of kinesin-1 motors onto these organelles for transport to the invadopodia periphery along microtubules. Synaptotagmin VII (SYT7) then mediates fusion of the LE with the PM. This serves a dual purpose in invadopodia maturation by (a) providing membrane to the growing invadopodia and (b) delivering MT1-MMP for exocytosis to degrade ECM. The first key finding of this study was that Protrudin is important for invadopodia maturation but dispensable for their formation. For this, invadopodia reformation was assayed in Protrudin-depleted MDA-MB-231 cells. The number of invadopodia formed upon serum repletion of starved cells was comparable to the number in control cells, indicating that the precursors do assemble in the absence of Protrudin (Fig. 1). However, the size of invadopodia was markedly reduced in Protrudin-depleted cells, suggesting that the structure fails to further elongate in the absence of Protrudin. Overexpression of Protrudin rescued the defect of invadopodia outgrowth supporting the role of Protrudin in the elongation phase of invadopodia.
The authors then proceed to dig deeper into the mechanism of Protrudin-mediated invadopodia elongation. LE-bound MT1-MMP is transported to invadopodia, where it is tethered to the invadopodial membrane via exocyst complex for fusion mediated by SNARE proteins. The authors hypothesized that Protrudin interacts with the MT1-MMP positive endosomes and facilitates their anterograde transport (Fig. 1). This was confirmed by TIRF imaging, where the number of MT1-MMP fusion events with the PM was much lower in Protrudin-depleted cells than in controls. The functional effect of reduced invadopodia elongation and MT1-MMP exocytosis was demonstrated as reduction in the degradation of ECM components, gelatin and collagen I, which translates to cells lacking invasion competence in vitro.
Intriguingly, overexpression of Protrudin in noncancerous RPE-1 cells mimicked the effects observed in cancerous cells. There was enhanced formation of invasive protrusions exocytosing more MT1-MMP. In this context, it would be important to determine the endogenous expression levels of Protrudin in cancerous versus noncancerous cells/tissues. This would help understand if invasive cancer cells generally display high Protrudin expression, and if this alone could confer an invasive phenotype. It is possible that additional signals in cancerous cells promote, even at moderate Protrudin expression levels, the number of events where Protrudin associates with the MT1-MMP-laden LE to drive their anterograde transport.
Finally, the authors seek cues of the clinical relevance of their findings by analyzing whether Protrudin expression level associates with the survival probability of cancer-afflicted patients by using publicly available databases. The findings demonstrate a negative correlation between survival rate and Protrudin expression. However, this interesting preliminary finding requires further research and must be interpreted with caution. The fascinating study by Pedersen et al. lays the foundation for future in vivo research to elucidate the role of Protrudin in tumor progression. The study significantly contributes to our understanding of the mechanisms that govern invadopodia function and inspires investigations aimed at finding new strategies to prevent cancer invasion and metastasis.
Acknowledgments
Mikko Olkkonen is thanked for preparing the figure.
The authors’ group is supported by the Academy of Finland (grant no. 322647 to V.M. Olkkonen), the Sigrid Juselius Foundation, the Finnish Foundation for Cardiovascular Research, and the Magnus Ehrnrooth Foundation.
The authors declare no competing financial interests.
References
- 1.Yamaguchi H., et al. . 2005. J. Cell Biol. 10.1083/jcb.200407076 [DOI] [Google Scholar]
- 2.Paterson E.K., and Courtneidge S.A.. 2018. FEBS J. 10.1111/febs.14123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oser M., et al. . 2010. J. Cell Sci. 10.1242/jcs.068163 [DOI] [Google Scholar]
- 4.Boateng L.R., and Huttenlocher A.. 2012. Eur. J. Cell Biol. 10.1016/j.ejcb.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eddy R.J., et al. . 2017. Trends Cell Biol. 10.1016/j.tcb.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poincioux R., et al. . 2009. J. Cell Sci. 10.1242/jcs.034561 [DOI] [Google Scholar]
- 7.Pedersen N.M., et al. . 2020. J. Cell Biol. 10.1083/jcb.202003063 [DOI] [Google Scholar]
- 8.Shirane M., and Nakayama K.I.. 2006. Science. 10.1126/science.1134027 [DOI] [Google Scholar]
- 9.Hashimoto Y., et al. . 2014. J. Biol. Chem. 10.1074/jbc.M113.528687 [DOI] [Google Scholar]
- 10.Raiborg C., et al. . 2015. Nature. 10.1038/nature14359 [DOI] [Google Scholar]