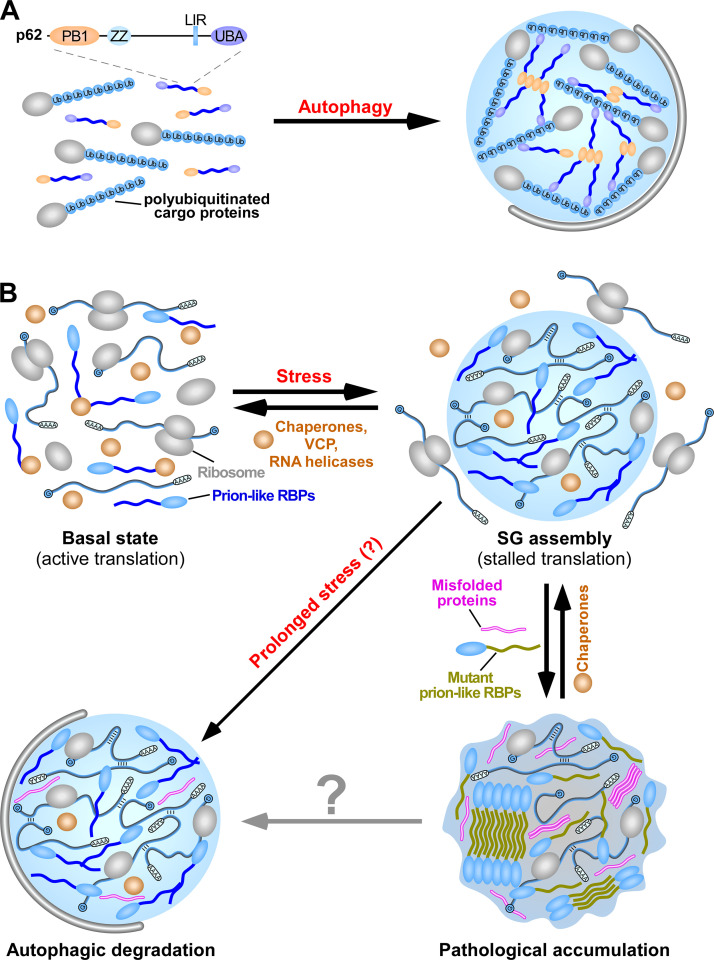
Figure 4.
Phase separation of p62-polyubiquitinated protein aggregates and SGs. (A) LLPS mediates the assembly of condensates containing p62 and polyubiquitinated proteins. p62 polymerizes and interacts with ubiquitin to drive LLPS. In the condensates, p62 forms filaments via PB1 polymerization, while the polyubiquitinated proteins cross-link the p62 filaments via ubiquitin–UBA domain interaction. p62 aggregates are selectively enclosed by autophagosomal membranes. LIR, LC3-interacting region; PB1, self-polymerization Phox and Bem1 (PB1) domain; UBA, UBA domain; ZZ, ZZ-type zinc-finger domain. (B) SGs are assembled via LLPS. SGs contain translationally stalled mRNAs, associated preinitiation factors, and various prion-like RBPs. Once the SG-inducing stress is mitigated, SGs rapidly disassemble with the assistance of chaperone proteins, VCP, and RNA helicases. A subset of SGs that fail to disassemble are subjected to autophagic degradation. Incorporation of misfolded proteins or mutations in SG-resident prion-like RBPs causes aberrant transition of SGs into solid fibrillar structures, resulting in their accumulation.