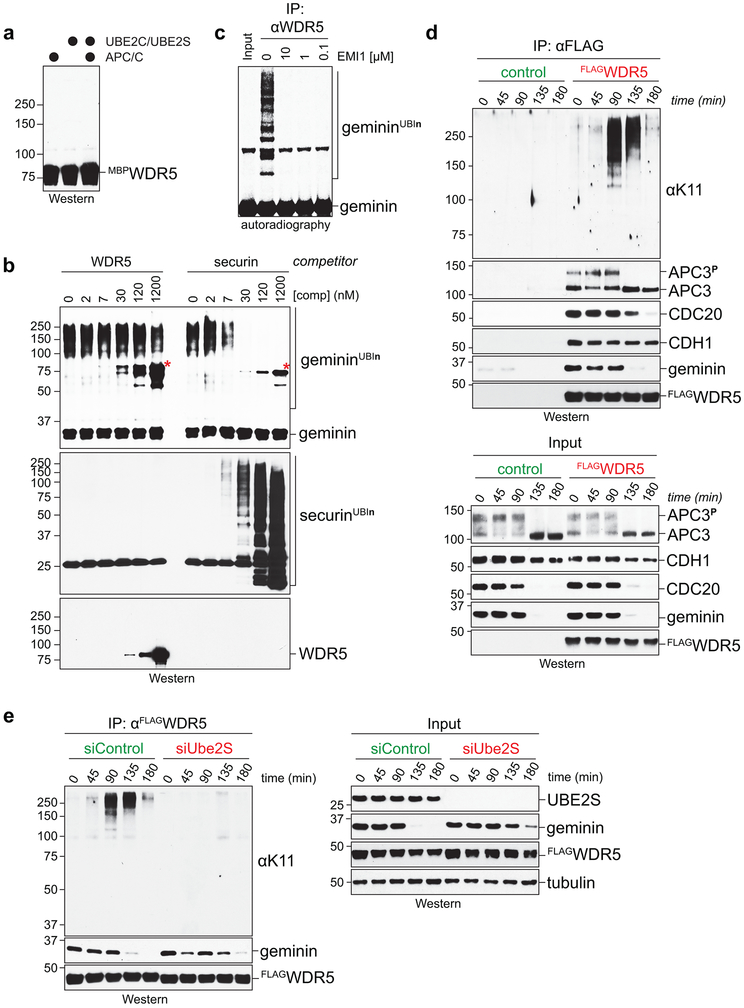
Extended Data Figure 6 ∣. WDR5 associates with active APC/C.
a, APC/C does not ubiquitylate WDR5 in vitro. Recombinant WDR5 was incubated with active APC/C, E1, UBE2C, UBE2S, and ubiquitin, and potential reaction products were detected by Western blotting against WDR5. This experiment was performed once.
b, APC/C-dependent ubiquitylation of geminin is outcompeted by recombinant securin (comp), a canonical substrate, but not by recombinant WDR5. Securin or WDR5 was added to APC/C-dependent geminin ubiquitylation reactions at the indicated concentrations, and various reaction products were detected using Western blotting. Asterisks represent cross-reactive bands. This experiment was performed once.
c, APC/CWDR5-dependent ubiquitylation of geminin is inhibited by EMI1. WDR5 affinity purifications from mitotic HeLa cells were incubated with E1, the APC/C-specific E2s UBE2C and UBE2S, and ubiquitin. EMI1 was added at indicated concentrations, and reaction products were detected by Western blotting using antibodies against geminin. This experiment was performed two independent times with similar results.
d, IP of FLAGWDR5 from mitotic HEK 293T cells co-precipitates K11-linked ubiquitin chains. 293T cells arrested in prometaphase were released into fresh medium, and WDR5 was affinity purified at indicated time points. Bound proteins were detected by Western blotting. This experiment was performed once.
e, Depletion of UBE2S eliminates WDR5-associated K11-linked ubiquitin chains in mitotic HEK 293T cells. This experiment was performed once.