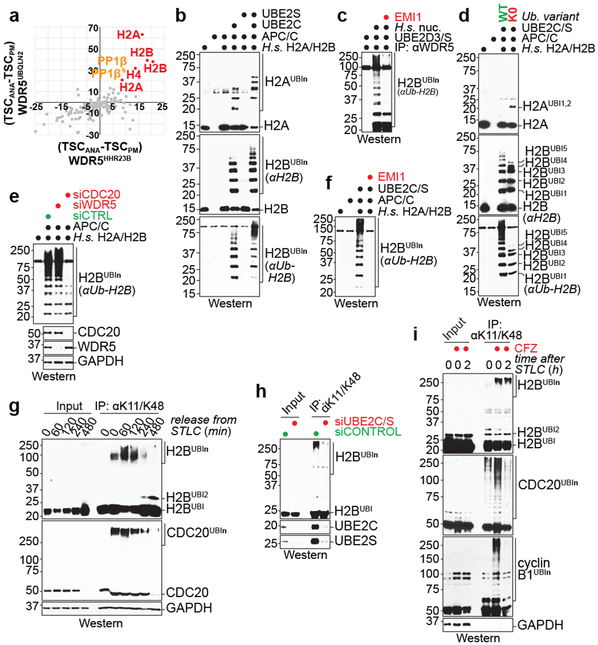
Fig 3. ∣. APC/CWDR5 decorates histone proteins with K11/K48-branched ubiquitin chains.
a, Mass spectrometry of WDR5HHR23B and WDR5UBQLN2 traps identifies histones as candidate substrates. Traps were affinity-purified from prometaphase (PM) or anaphase (ANA) HeLa cells, with low or high APC/C activity, respectively.
b, APC/CCDC20 purified from mitotic HeLa S3 cells ubiquitylates recombinant human H2A/H2B dimers. This experiment was performed four independent times with similar results.
c, APC/CWDR5 ubiquitylates H2B in polynucleosomes purified from HeLa cells and is inhibited by the APC/C inhibitor EMI1. This experiment was performed three independent times with similar results.
d, APC/CWDR5 ubiquitylates multiple Lys residues in histones, as seen with Lys-free ubiquitin (K0). This experiment was performed two independent times with similar results.
e, APC/C-dependent ubiquitylation of H2B requires CDC20 in vitro. APC/C was purified from mitotic HeLa cells depleted off CDC20 or WDR5. This experiment was performed once.
f, Ubiquitylation of H2B by the APC/C is dependent on UBE2C and UBE2S and inhibited by EMI1. This experiment was performed two independent times with similar results.
g, Endogenous H2B is modified with K11/K48-branched chains, as seen by denaturing purification from synchronized HeLa cells. This experiment was performed three independent times with similar results.
h, Mitotic K11/K48-modification of endogenous H2B in hESCs is dependent on UBE2C and UBE2S. This experiment was performed two independent times with similar results.
i, Proteasome inhibition stabilizes mitotic K11/K48-modified H2B in H1 hESCs. This experiment was performed two independent times with similar results.