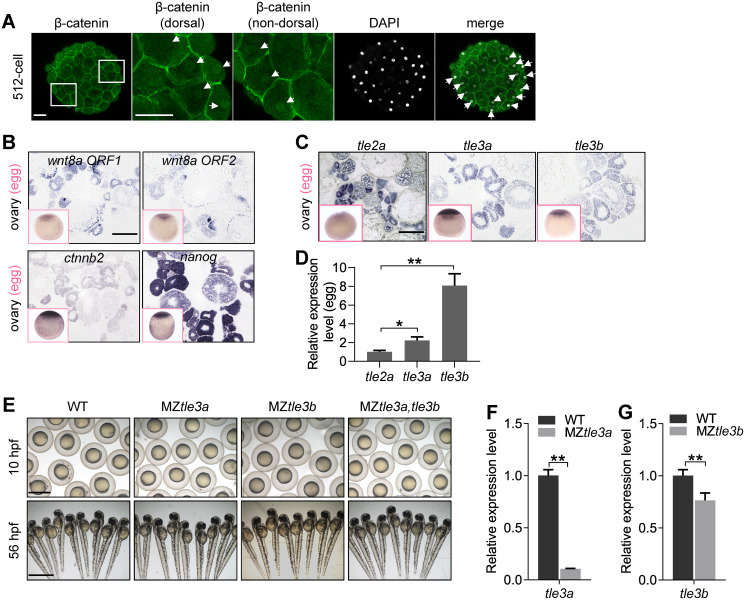
Fig 1. Maternal TLEs do not likely contribute to the repression of maternal β-catenin activity.
(A) Detection of nuclear localization of maternal β-catenin in embryo at 512-cell stage by immunostainning against β-catenin. Signals were observed at animal view. Nuclei were co-stained with DAPI. Arrow heads indicate the nuclear accumulation of β-catenin. Scale bar, 50 μm. (B) In situ hybridization on cryosections of ovaries and WISH on unfertilized eggs (pink framed squares) showing wnt8a1, wnt8a2, ctnnb2, and nanog are maternally expressed during oogenesis and in unfertilized eggs. Scale bar, 100 μm. (C) In situ hybridization on cryosections of ovaries and WISH analysis of unfertilized eggs (pink framed squares) showing tle2a, tle3a, and tle3b are maternally expressed during oogenesis and in unfertilized eggs. Scale bar, 100 μm. (D) In comparison with tle2a, tle3a, and tle3b are significantly highly expressed in matured eggs as shown by RT-qPCR analysis. Error bars, mean ± SD, *P < 0.05, **P < 0.01. (E) The maternal -zygotic mutant of tle3a (MZtle3a) or tle3b (MZtle3b), or double mutant of tle3a and tle3b (MZtle3a, tle3b), showed no early developmental defect. Scale bar, 1 mm. (F) RT-qPCR analysis showing mRNA expression level of tle3a was significantly reduced in MZtle3a at 3 hpf. Error bars, mean ± SD, **P < 0.01. (G) RT-qPCR analysis showing mRNA expression level of tle3b was significantly reduced in MZtle3b at 3 hpf. Error bars, mean ± SD, **P < 0.01. The P values in this figure were calculated by Student t test. The underlying data in this figure can be found in S1 Data. hpf, hours post fertilization; MZtle3a, maternal -zygotic mutant of tle3a; MZtle3b, maternal -zygotic mutant of tle3b; RT-qPCR, reverse-transcription quantitative PCR; TLE, transducin-like enhancer of split; WISH, whole-mount in situ hybridization; WT, wild type.