Abstract
Pediatric patients with differentiated thyroid cancer (DTC) tend to have more advanced disease at presentation, for which more aggressive radioiodine (RAI) treatment would be commonly recommended. Several previous studies recommended dosimetry to calculate the optimal activity of RAI in pre-pubertal children and in children with a significant distant metastasis. This study aimed to evaluate the effect of empirical RAI treatment for DTC on bone marrow function in pre-pubertal children and adolescents.
DTC patients aged ≤ 18 years receiving empirical RAI treatment with complete blood count data before and after treatment were included and divided into pre-pubertal and pubertal groups. Blood count values at baseline and 1.5–3 months, 3–6 months, and 6–12 months after RAI treatment were compared. The effect of demographic, clinical, and laboratory variables on bone marrow function were assessed.
We included 83 patients (113 treatments). At diagnosis, pre-pubertal children had more aggressive tumor features, including tumor size (P = .045) and distant metastases (P = .037). Approximately 51% to 96% of hypocellular bone marrow, and 11% to 14% of anemia were observed in the pre-pubertal and pubertal groups, with a majority of mild (Grade 1–2) and minority of moderate (Grade 3) bone marrow suppression. No significant differences in bone marrow function or Common Terminology Criteria for Adverse Events (CTCAE) grades were found between the pre-pubertal and pubertal groups after RAI treatment. None of the clinical factors tested were found to be significant predictors for bone marrow suppression after RAI treatment.
Empirical RAI treatment for DTC in pre-pubertal children and adolescents causes mild to moderate bone marrow suppression with limited clinical significance. With adequate preparations for RAI treatment, empirical high activities (150–200 mCi) could be safe and well tolerated by both pre-pubertal and pubertal patients with DTC.
Keywords: empirical radioiodine treatment, bone marrow function, children and adolescents, differentiated thyroid cancer, adverse events
1. Introduction
Pediatric differentiated thyroid cancer (DTC) is one of the most common childhood endocrine malignancies, accounting for approximately 4.6% of all cancers in children aged ≤ 14 years and for 7.4% of cancers in adolescents aged 15 to 19 years, with a preponderance in girls.[1,2] According to the Surveillance, Epidemiology and End Results (SEER) program, 1.8% of new thyroid cancers were reported in the United States in patients ≤ 20 years of age between 2012 and 2016.[3] Moreover, the Report of Cancer Epidemiology in China revealed that thyroid cancer in female patients represented 3.8% of all new cancer cases in 2015, with approximately 151,000 new cases of female thyroid cancer (the 4th most common tumor).[4]
Compared with adults, several studies have evaluated DTC in the pediatric population, revealing that pediatric patients with DTC are more prone to extra-thyroidal extension, regional lymph node involvement, and pulmonary metastasis.[5–9] Furthermore, pediatric DTC patients are at a risk of very high overall recurrence rates of nearly 30%.[10–13] Moreover, several studies reported more aggressive presentation and worse outcomes in pre-pubertal patients than in pubertal ones.[14–17]
It is well-known that children are more sensitive to radiation, which results in a better response to RAI therapy; however, they are at a higher risk of acute and long-term harms from over-aggressive treatment, such as acute bone marrow suppression and second malignancy.[18–21] Tuttle et al observed that the empirical dosing strategy resulted in the administered RAI activities exceeding the maximum tolerable activity (MTA) safety limit of 200 cGy defined for bone marrow in many patients with metastatic DTC.[22] Furthermore, they found that an empirically administered RAI activity of 200 mCi would exceed the MTA in 14% of patients <19 years and the administration of 250 mCi would exceed the MTA in 20% of patients <19 years.[22] Considering the safety of RAI therapy in pediatric population, several dosimetry-based studies (including blood-based and lesion-based dosimetry approaches) have been performed. These studies have shown that in children with a significant distant metastasis or limited bone marrow reserve and in pre-pubertal children, dosimetry should be considered to calculate the optimal activity of RAI. [23–26]
In 2017, Albano et al studied data from 302 RAI treatments in 105 DTC patients aged ≤18 years and found 11 cases (6 of Grade 1 and 5 of Grade 2 according to the CTCAE classification) involving transient bone marrow suppression and 4 cases (2 in grade 1 and 2 in grade 2) involving permanent bone marrow suppression.[27] They also noted that transient bone marrow suppression was associated with the amount of administered activities in each treatment, while permanent bone marrow suppression was correlated with the number of treatments and cumulative activities of RAI.[27] However, they did not compare the bone marrow function between pre-pubertal children and adolescents, neither did they investigate the relationship between bone marrow suppression and the renal function. Moreover, the 2014 American Thyroid Association (ATA) guidelines on children with thyroid cancer recommended that “pre-pubertal” and “pubertal/post-pubertal” should be incorporated into future studies to increase uniformity and more accurately represent the potential influence of pubertal development on the behavior of DTC within the pediatric population.[28]
Given the higher incidence, the more extensive presentation, the higher risk of side effects caused by RAI treatment, increased concern with RAI dosimetry in DTC pediatric patients, and the ATA recommendation on pre-pubertal and pubertal groups, we sought to evaluate the data available for DTC patients at West China Hospital of Sichuan University. Our study is the first to compare the effect of RAI therapy on bone marrow function between pre-pubertal and pubertal DTC patient cohorts, and to investigate the relationship between bone marrow suppression and the renal function in pediatric patients. In the present study, we aimed to investigate the following:
-
1)
the effect of various empirically administered RAI activities on bone marrow function in pre-pubertal children and adolescents; and
-
2)
the relationship between bone marrow suppression after RAI treatment and multiple factors including sex, age, the single administered RAI activity, cumulative activity, total number of RAI treatments, intervals between repeated treatments, and renal function in patients ≤18 years of age.
2. Methods
The study protocol was approved by the Institutional Research Ethics Committee of West China Hospital of Sichuan University (# 2019671), and the requirement of written informed consent was waived.
2.1. Patients
The electronic medical records at West China Hospital of Sichuan University were retrospectively reviewed for all pediatric patients with DTC who were treated with RAI for remnant ablation, adjuvant treatment, or treatment of known disease between July 2007 and February 2019. The inclusion criteria were as follows: patients aged ≤18 years at the time of RAI treatment[28]; all pediatric patients with documented DTC who underwent total thyroidectomy or near-total thyroidectomy, central node dissection, and/or lateral neck dissection, postoperative RAI treatment, and thyroid-stimulating hormone (TSH) suppressive therapy; access to electronic medical records; those who has undergone renal function test before RAI treatment; and those who had undergone complete blood count (CBC) before and more than 1 year after RAI treatment. Inclusion was per treatment (and not per patient), as patients could have received several treatments. Some cases were excluded depending on the data available, for example, treatments without sufficient data on electronic medical record, renal function, or CBC. The activity of RAI was based on empirical dosing defined by the treating physician. In cases where several RAI treatments were prescribed with some before the patient has reached 18 years of age and some after, some belonging to the pre-puberty and some to the puberty groups, only the treatments applied when the patients were below 18 years of age and belonging to pre-puberty or puberty groups were included with data of the number of previous treatments and the cumulative dose. Exclusion criteria were histopathological diagnoses other than DTC, active concomitant malignancy, current treatment with chemotherapy, kinase inhibitors or immunotherapy, and hematological disease.
In this study, all the pediatric patients were divided into the pre-puberty and puberty groups according to their pubertal stages (girls’ menarche and boys’ first emission) at each RAI treatment. More specifically, girls after menarche were classified as the pubertal group, while others were classified as pre-pubertal.[29] According to the “Report on the Physical Fitness and Health Surveillance of Chinese School Students (1991)”, the mean age of boys’ first emission was 13.84 years of age in Beijing.[30] Therefore, we classified boys aged ≥14 years as the pubertal group, while the younger boys were classified as the pre-pubertal group.
2.2. Data Collection
The following data were collected: age at the time of treatment; sex; tumor size and histology; tumor-node-metastasis (TNM) stage according to the American Joint Committee on Cancer (AJCC) staging manual eighth edition[31]; current RAI activity; cumulative RAI activity; renal function (e.g., blood creatinine, urea level and the estimated glomerular filtration rate [eGFR]); and CBC before and after RAI treatment, including red blood cell (RBC), hemoglobin (Hb), platelet (PLT), white blood cell (WBC), neutrophil (NEUT), lymphocyte (LYMPH). Additional data were collected for hospitalizations, acute infections, blood transfusions, or treatment with granulocyte colony-stimulating factor within 1 year following RAI treatment. Bone marrow suppression was defined as the suppression of bone marrow function as a result of significant reduction in the number of RBC, Hb, PLT, WBC, NEUT, and LYMPH according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.[32]
Whole-blood samples were processed by the Sysmex XE-5000 analyzer (Sysmex Corp., Kobe, Japan). Intervals assessed for bone marrow function were as follows: baseline values (within 3 months before RAI), 1.5–3 months, 3–6 months, 6–12 months after RAI treatment.
2.3. Statistical Analysis
Statistical calculations were performed using IBM SPSS statistical software (SPSS version 23.0 for Mac OS X). Continuous descriptive variables were presented as means ± standard deviation (SD), and categorical variables were calculated as frequencies or percentages. The association between clinicopathological characteristics and pre-puberty/puberty was evaluated with either the independent-sample t-test, χ2 test, or Fisher exact test. A paired-sample t test was used to compare CBC values at each time point with the baseline values. Then, the bone marrow function with/without suppression was compared between the pre-pubertal and adolescent groups using χ2 test or Fisher exact test. Furthermore, the Mann-Whitney nonparametric U-test was performed to analyze the different CTCAE grades of bone marrow suppression in the 2 groups. The significance of multiple clinical factors associated with bone marrow suppression following RAI treatment was assessed using logistic regression analysis. A P value of <.05 was considered as statistically significant.
3. Results
3.1. Patients
A total of 187 RAI treatments in 103 DTC patients who were ≤18 years old at the time of treatment were eligible for analysis. Among these, 74 RAI treatments were excluded due to the following: missing data (n = 48); cross puberty (n = 16); >18 years old at the time of RAI treatment (patients who had two or more RAI treatments, of which some were done when patients were ≤ 18 years old and some when patients were >18 years old; n = 5); or concomitant cancer treatment (n = 5). Finally, in total, 113 RAI treatments in 83 patients met the inclusion criteria and were analyzed (Fig. 1).
Figure 1.
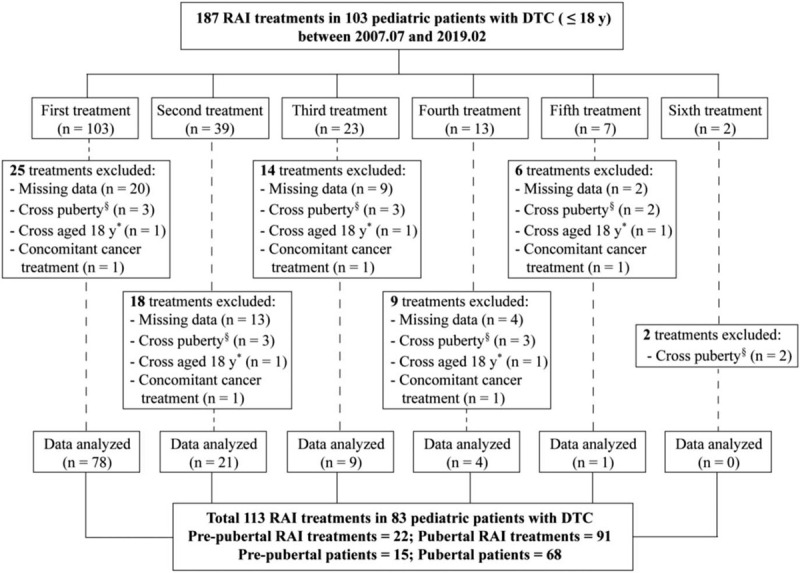
Flow chart of inclusion-exclusion of pediatric patients with DTC at the study. ∗In cases where several RAI treatments were prescribed with some belonging to the pre-puberty and some to the puberty groups. §In cases where several RAI treatments were prescribed with some before the patient has reached 18 years of age and some after. DTC = differentiated thyroid cancer, RAI = radioiodine.
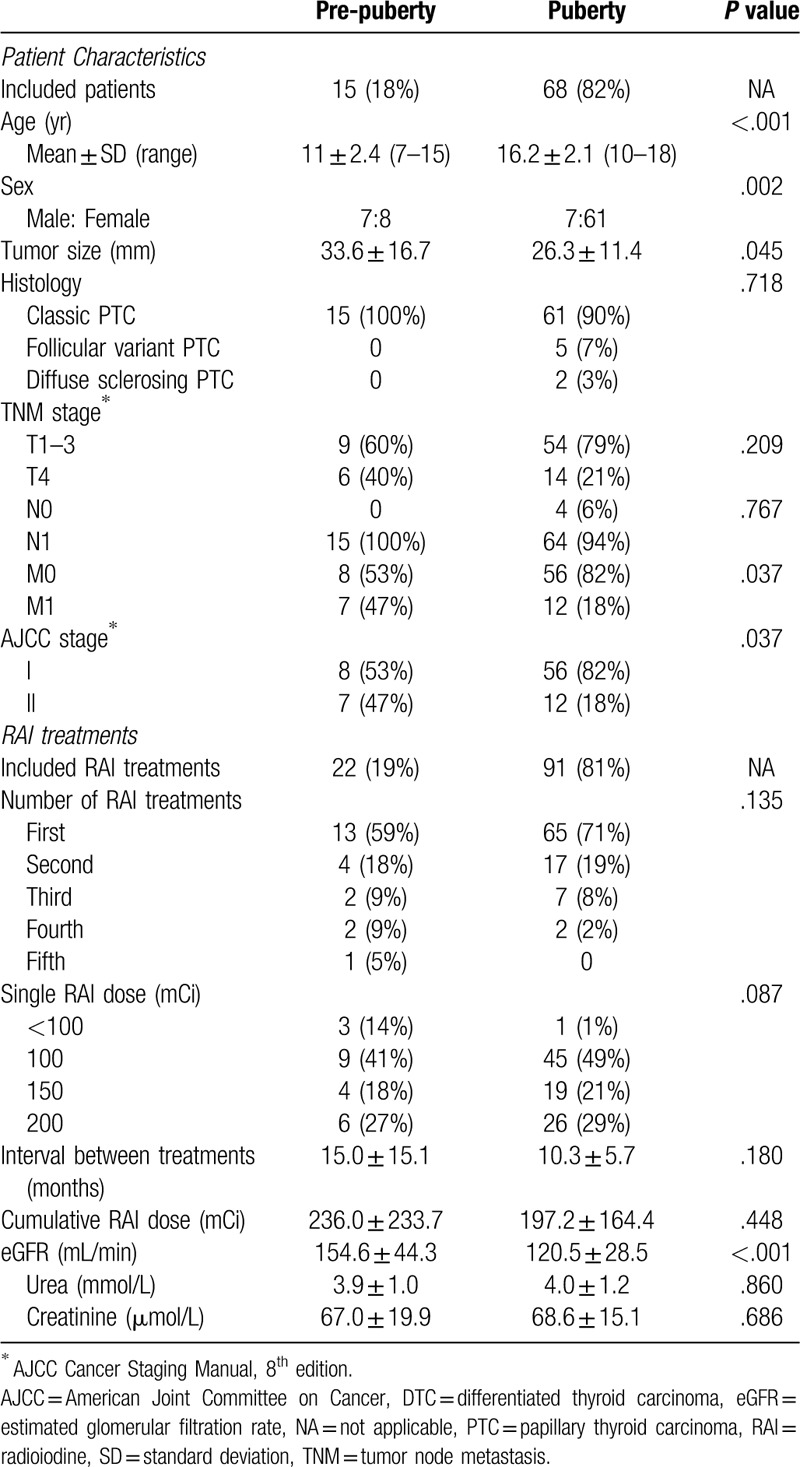
Of the total 113 RAI treatments in 83 pediatric patients, 22 treatments in 15 patients were administered at a pre-pubertal age and 91 treatments in 68 patients were administered at a pubertal age (Table 1). The mean age at the RAI treatment was much lower in the pre-pubertal children than that in the pubertal ones (P < .001). There was a preponderance of female patients in the adolescent group, compared with that in the pre-pubertal group (90% vs 53%; P = .002). The mean primary tumor size was significantly higher in the pre-pubertal group than in the pubertal one (P = .045). Regarding tumor histology, classical papillary thyroid carcinoma (PTC) was identified in all of the pre-pubertal children and in 90% (61/68) of the adolescents. Extensive tumor features at presentation were more prevalent in the pre-pubertal children than in the pubertal group. T4 disease (T4a, tumor of any size extending beyond the thyroid capsule to invade subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve; T4b, tumor of any size invading prevertebral fascia or encasing carotid artery or mediastinal vessels) was found in 40% (6/15) of the pre-pubertal patients but in only 21% (14/68) of the pubertal ones. Lymph node metastases were observed in all pre-pubertal patients and in 94% (64/68) of the pubertal patients. Distant metastases and stage II disease, according to the AJCC staging manual eighth edition, were present in 47% (7/15) of the pre-pubertal children while only in 18% (12/68) of the pubertal patients (P = .037).
Table 1.
Clinicopathological characteristics of DTC in 83 pediatric patients with 113 RAI treatments—comparison between pre-pubertal children and adolescents.
3.2. RAI treatment
Of the 22 RAI treatments in pre-pubertal children analyzed, 59% (13/22) had their first treatment, 18% (4/22) - second treatment, 9% (2/22) - third treatment, 9% (2/22) - fourth treatment, and 5% (1/22) - fifth treatment; for the 91 RAI treatments in the pubertal group, 71% (65/91) had their first treatments, 19% (17/91) - second treatments, 8% (7/91) - third treatments, and 2% (2/91) - fourth treatments (Table 1). A low activity of < 100 mCi was administered in only 14% (3/22) and 1% (1/91) of the treatments in the pre-pubertal and pubertal groups, respectively. Higher activities were administered in 86% (19/22) of the treatments in pre-pubertal group as follows: 100 mCi in 41% (9/22) of the patients; 150 mCi in 18% (4/22) of the patients; and 200 mCi in 27% (6/22) of the patients. For 99% (90/91) of treatments in adolescents, the proportions were 49% (45/91), 21% (19/91), and 21% (19/91), respectively. The mean intervals between repeated treatments, mean single administered RAI activity, mean cumulative activity, and renal function at the time of the treatment (including urea, serum creatinine, and estimated glomerular filtration rate [eGFR]) are presented in Table 1. All patients were prepared for the RAI treatments by thyroid hormone withdrawal (THW); patients who were administered high activities (150–200 mCi) were prepared through 1 to 2 weeks of prophylactic treatment for leukopenia and thrombocytopenia before and after RAI treatment, and by maintaining optimal intervals between repeated treatments. There was no comorbidity in both groups.
3.3. Bone marrow function
For pre-pubertal children at the time period of 1.5 to 3 months after RAI treatment, there was a slight but statistically significant decrease in the count of RBC (8.0% decrease; P < .001), Hb (6.5% decrease; P < .001), and WBC (20.4% decrease; P = .003); for pubertal group, the bone marrow suppression measurements were: RBC (6.4% decrease; P < .001), Hb (5.3% decrease; P < .001), PLT (5.2% decrease; P = .020), and WBC (13.1% decrease; P < .001; Table 2). The decrease in WBC counts was mostly due to the reduction in LYMPH counts: 43.5% decrease in the pre-pubertal group (P < .001) and 34.8% decrease in the pubertal (P < .001) group, with no significant change in neutrophil counts. On the subgroup analyses, the most sensitive type of blood cell was WBC, which showed sharp decrease after treatment with ≥ 100 mCi, especially in the pre-pubertal children as compared with that in pubertal group (17.3% vs 13.1% decrease; Table 2). Although there was a slight decrease in the counts of RBC, Hb, PLT, and WBC in the group of pre-pubertal children treated with ≥ 150 mCi, it is plausible that because of the relatively small cohort (n = 10), the statistical power was insufficient to detect the significant CBC decrease. In contrast, bone marrow suppression in the adolescent group treated with ≥ 150 mCi (n = 45) was significant as detected by the reduction in RBC (6.4% decrease; P < .001), Hb (4.4% decrease; P < .001), PLT (6.6% decrease; P = .022), WBC (14.5% decrease; P < .001), and LYMPH (36.4% decrease; P < .001). Further analysis of bone marrow suppression in pre-pubertal children and adolescents treated with 100 mCi, 150 mCi, and 200 mCi is presented in Table 2. Both in the pre-pubertal and pubertal groups, RBC, Hb, PLT, WBC, and LYMPH levels gradually recovered after 3 to 6 months of RAI treatment (Fig. 2). None of the patients, including the 4 pre-pubertal children with a mean age of 10.8 years (range 9–13 years) with 6 RAI treatments who received 200 mCi, required treatment for cytopenia with colony-stimulating factors, or blood transfusions during the first year of follow-up.
Table 2.
Comparison of CBCs at baseline and CBCs after 1.5–3 months of RAI treatment according to puberty.
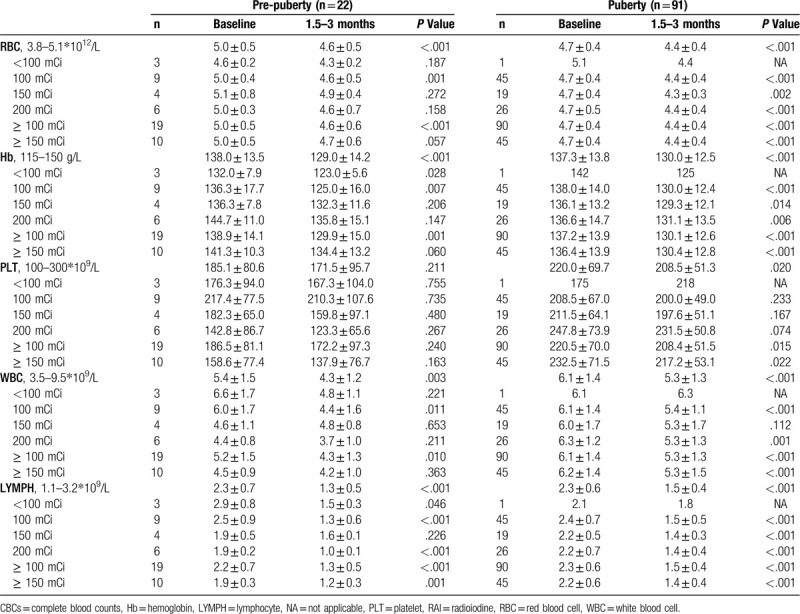
Figure 2.
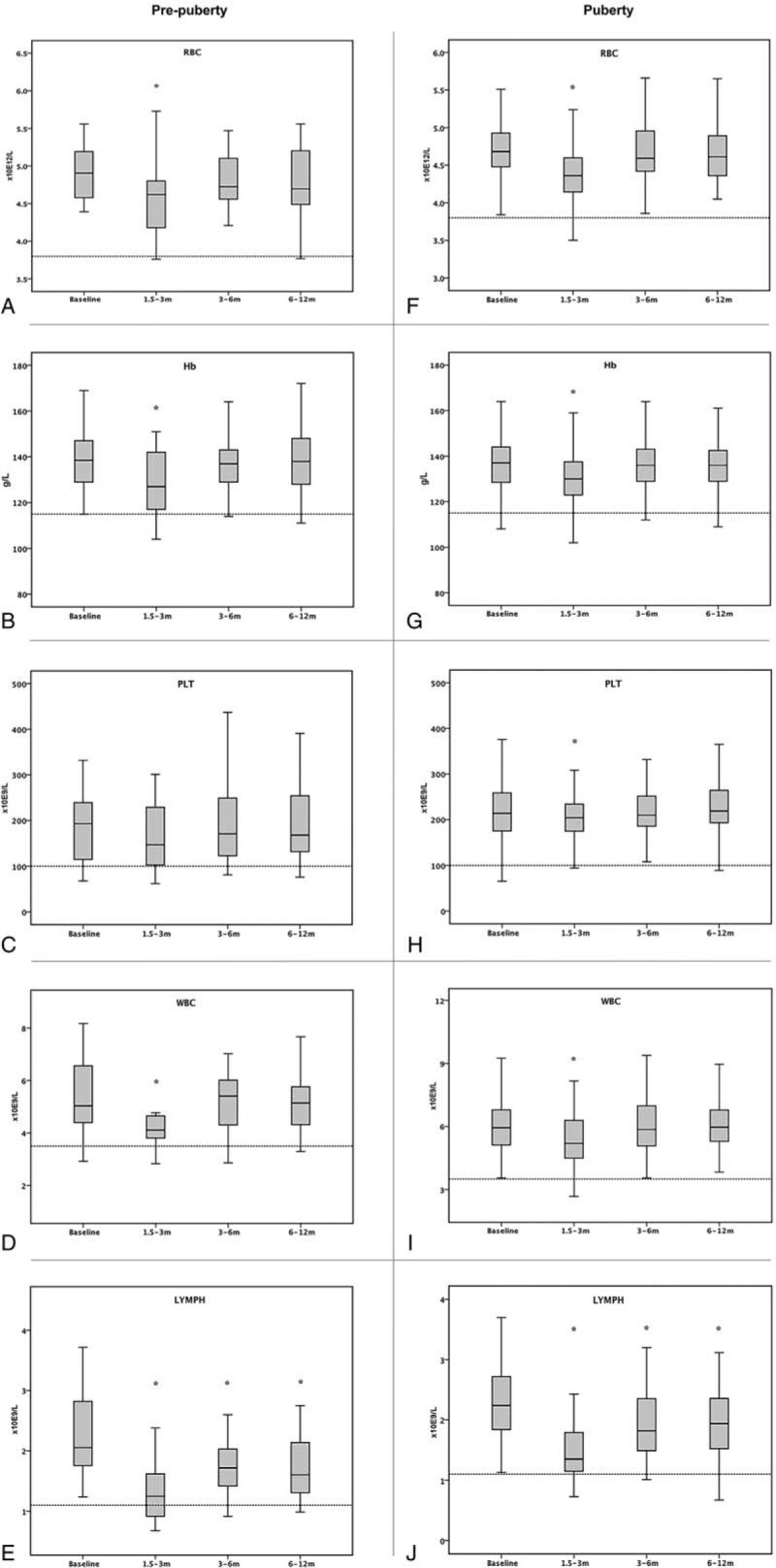
Blood count values at baseline and 1.5–3 months, 3–6 months, and 6–12 months after RAI treatment in pre-pubertal and pubertal groups. ∗Blood counts decreased compared to baseline (P < .05).
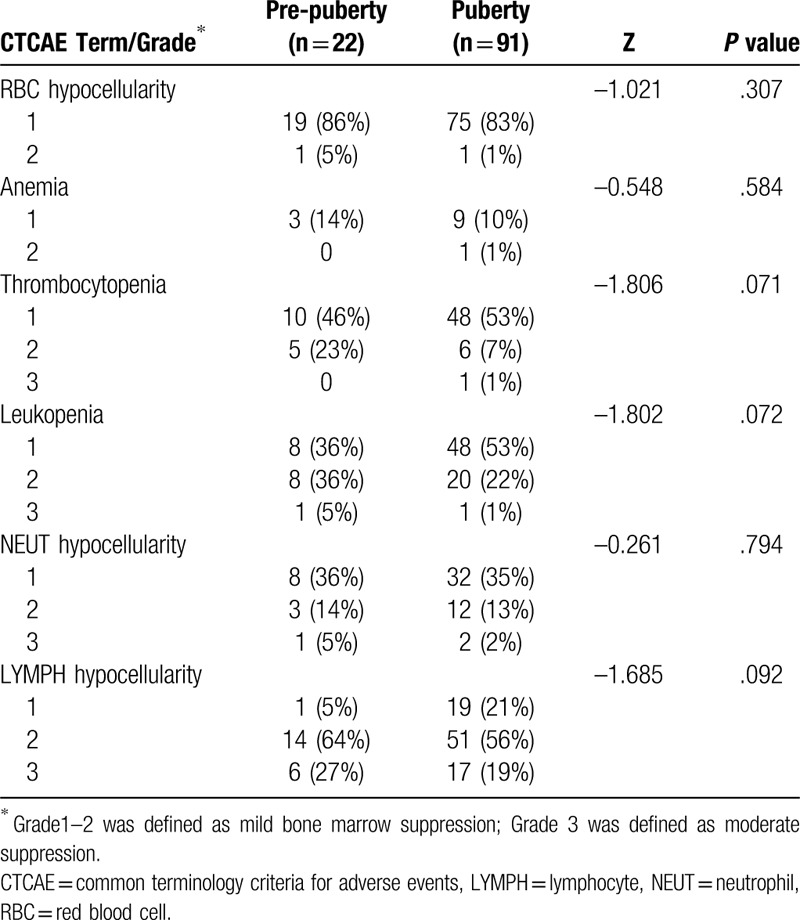
Bone marrow suppression occurring within 1.5 to 3 months of RAI treatment according to CTCAE version 5.0 (as compared with baseline levels) both in the pre-pubertal and pubertal groups is shown in Tables 3 and 4. Approximately 51% to 96% of hypocellular bone marrow, and 11% to 14% of anemia were observed in the pre-pubertal and pubertal groups, with a majority of mild (Grade 1–2) and minority of moderate (Grade 3) bone marrow suppression. However, no significant differences in the bone marrow function after RAI treatment were observed between the two groups (Table 3). Further analyses for the different CTCAE grades in each blood cell type in the cohort revealed that the majority of bone marrow suppression was mild (Grade 1–2), with a minority showing moderate suppression (Grade 3). Similarly, there was no significant difference in CTCAE grades detected in each blood cell type between pre-pubertal children and adolescents (Table 4).
Table 3.
Comparison of bone marrow suppression (according to CTCAE v.5.0) between the pre-pubertal and pubertal groups.
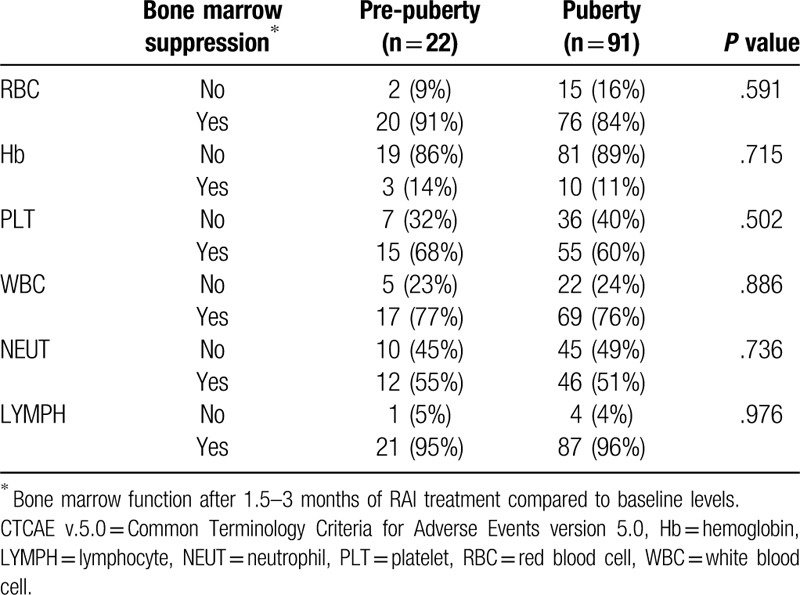
Table 4.
Comparison of CTCAE grades of bone marrow suppression between the pre-pubertal and pubertal groups.
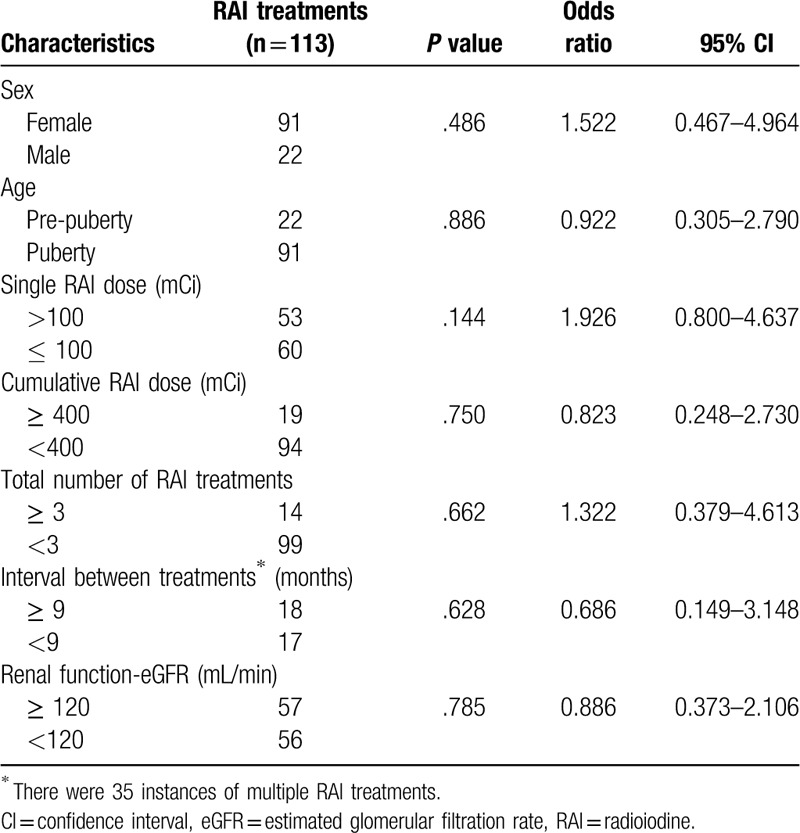
The correlations between the patients’ characteristics, including sex, age, single RAI dose, cumulative RAI dose, the total RAI treatments, intervals between repeated treatments, renal function (eGFR) at the time of the treatment, and bone marrow suppression are presented in Table 5. In the logistic regression analysis, none of the factors tested was found to be significant predictors of bone marrow suppression in the univariate analyses.
Table 5.
Univariate analysis of the correlations between patient characteristics and bone marrow suppression.
4. Discussion
In this study, we evaluated the effect of the empirical RAI treatment on bone marrow function in pediatric patients with DTC. We compared for the first time the CBC count between 2 groups: the pre-pubertal and pubertal patients at different time points: starting from within 3 months before the RAI treatment and up to 1 year after the RAI treatment. We observed no significant differences between the pre-pubertal and pubertal groups in either the bone marrow function after the RAI treatment, or in the different CTCAE grades in each blood cell type. Moreover, in this study, none of the clinical factors were found to be significant predictors for bone marrow suppression after RAI treatment in pediatric patients with DTC.
Specifically, our study revealed that between 1.5 and 3 months after RAI treatment, approximately 51% to 96% of hypocellular bone marrow, and 11% to 14% of anemia were observed in the pre-pubertal and pubertal groups, with a majority of mild (Grade 1–2) and minority of moderate (Grade 3) bone marrow suppression. The grade of bone marrow suppression in our study was consistent with previously published data showing the mainly mild bone marrow suppression after RAI treatment.[27,33–35] However, our findings revealed a much higher rate of bone marrow suppression in pediatric patients than that reported in a previous study, which found 11 (6 of Grade 1 and 5 of Grade 2) cases of transient bone marrow suppression in 105 pediatric DTC cases with 302 RAI treatments.[27] A possible explanation for this discrepancy might be the timing. Bone marrow suppression usually occur within 1 to 2 months after RAI treatment.[33] The previous study, however, evaluated bone marrow function within 1 week after the treatment, which may have been too short to detect the reduction. In this study, we assessed bone marrow suppression within 1.5 to 3 months after treatment, when the effect was already more apparent.
Our study is the first to separately evaluate and compare bone marrow function after RAI therapy in pre-pubertal and pubertal DTC patient cohorts. Although an earlier study reported that pre-pubertal DTC patients had a more aggressive presentation than pubertal ones,[17] in our study, we found that there were no significant differences in either bone marrow function or the different CTCAE grades between the pre-pubertal and pubertal groups after RAI treatment. Moreover, in contrast to the earlier findings,[27,34,35] we could not detect the association between bone marrow suppression after RAI treatment and clinical factors including sex, age, tumor stage, single RAI dose, cumulative dose, and total number of RAI treatments in pediatric DTC patients. The reason for this discrepancy is not clear but it may be caused by the limited sample size of the pre-pubertal group (only 22 RAI treatments in 15 pre-pubertal children).
This study has 3 limitations. First, the puberty criteria we used can differ slightly from the current standard. The current internationally endorsed standard of defining puberty is the Tanner stage, originating from the Harpenden Growth Study by Marshall and Tanner in England during the 1960s–1970s.[36,37] They collected mixed longitudinal data on the physical changes at puberty in 192 normal girls[36] and 228 boys[37] which were characterized according to normal standards for 5 stages of breast, genitalia, and pubic hair development. Usually, Tanner stage 1 is defined as pre-puberty, while Tanner stages 2–5 are defined as puberty.[17] Given the retrospective design of this study, we could not evaluate the longitudinal development of breast, genitalia, and pubic hair in these patients. Therefore, we divided pediatric patients into pre-puberty and puberty groups according to girls’ menarche and boys’ first emission as presented in the Methods section. Although menarche or first emission serve as only a single event in the combination of secondary sex characteristic changes at puberty, the development of breasts, genitalia, and pubic hair and the adolescent growth spurt occur almost simultaneously lasting for approximately 3 years, with menarche and first emission emerging generally in the latter half of this period.[36,37] Second, the limited sample size used in this study was not optimal, especially in the pre-pubertal group, which had the following limitations:
-
1)
the different CTCAE grades in thrombocytopenia and leukopenia were not significantly associated with the pre-puberty/puberty;
-
2)
none of the risk factors were shown as a significant predictor of bone marrow suppression in the univariate analyses.
It is possible that with a larger data set, the different CTCAE grades in thrombocytopenia and leukopenia would be significantly associated with the pre-puberty/puberty, and the risk factors such as cumulative RAI dose or total number of RAI treatments would predict bone marrow suppression for pediatric patients. Finally, we could not evaluate the risk of long-term hematologic toxicity in the 1 year follow-up. As reported in previous study, it took 5 years for the WBC and PLT counts to recover to baseline levels after RAI treatment.[35]
In conclusion, we showed that empirical RAI treatment for DTC in both pre-pubertal children and adolescents cause mild to moderate bone marrow suppression with limited clinical significance. With adequate preparations for RAI treatment, empirical high activities (150–200 mCi) of RAI could be safe and well tolerated for both pre-pubertal and pubertal DTC patients. Future studies should define pre-puberty and puberty groups according to the accurate evaluation of the longitudinal development of breasts, genitalia, and pubic hair in pediatric patients and perform a long-term follow up.
Author contributions
Formal analysis: Ping Dong.
Funding acquisition: Ping Dong, Lin Li.
Investigation: Ping Dong, Li Wang, Rui Huang.
Resources: Ping Dong, Li Wang.
Software: Ping Dong, Li Wang.
Supervision: Rui Huang, Lin Li.
Visualization: Rui Huang, Lin Li.
Writing – original draft: Ping Dong, Li Wang.
Writing – review & editing: Rui Huang, Lin Li.
Footnotes
Abbreviations: CBC = complete blood count, DTC = differentiated thyroid cancer, CTCAE = common terminology criteria for adverse events, eGFR = estimated glomerular filtration rate, Hb = hemoglobin, LYMPH = lymphocytes, NEUT = neutrophil, MTA = maximum tolerable activity, PLT = platelet, RAI = radioiodine, RBC = red blood cell, WBC = white blood cell.
How to cite this article: Dong P, Wang L, Huang R, Li L. Bone marrow suppression in pediatric patients with differentiated thyroid cancer following empirical radioiodine therapy. Medicine. 2020;99:31(e21398).
This study was supported by the China Postdoctoral Science Foundation (No. 2019M663512) and the “1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYGD18016).
The authors declare no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Bernstein L, Gurney JG. Ries LAG, Smith MA, Gurney JG, et al. Carcinomas and other malignant epithelial neoplasms. ICCCXI. Pediatric Monograph. Cancer Incidence and Survival Among Children and Adolescents. United States SEER Program 1975–1995. Maryland: Bethesda; 1999. 3. [Google Scholar]
- [2].Qian ZJ, Jin MC, Meister KD, et al. Pediatric thyroid cancer incidence and mortality trends in the United States, 1973-2013. JAMA Otolaryngol Head Neck Surg 2019;145:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Thyroid Cancer. 2017;Bethesda, Maryland: National Cancer Institute, Available from: http://seer.cancer.gov/statfacts/html/thyro.html. Accessed September 23, 2019. [Google Scholar]
- [4].Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi 2019;41:19–28. [DOI] [PubMed] [Google Scholar]
- [5].Jain NK, Mostoufi-Moab S, Hawkes CP, et al. Extrathyroidal extension is an important predictor of regional lymph node metastasis in pediatric differentiated thyroid cancer. Thyroid 2019;doi:10.1089/thy.2019.0229. [DOI] [PubMed] [Google Scholar]
- [6].Alzahrani AS, Alswailem M, Moria Y, et al. Lung metastasis in pediatric thyroid cancer: radiological pattern, molecular genetics, response to therapy, and outcome. J Clin Endocrinol Metab 2019;104:103–10. [DOI] [PubMed] [Google Scholar]
- [7].Jeon MJ, Kim YN, Sung TY, et al. Practical initial risk stratification based on lymph node metastases in pediatric and adolescent differentiated thyroid cancer. Thyroid 2018;28:193–200. [DOI] [PubMed] [Google Scholar]
- [8].Sung TY, Jeon MJ, Lee YH, et al. Initial and dynamic risk stratification of pediatric patients with differentiated thyroid cancer. J Clin Endocrinol Metab 2017;102:793–800. [DOI] [PubMed] [Google Scholar]
- [9].Parisi MT, Mankoff D. Differentiated pediatric thyroid cancer: correlates with adult disease, controversies in treatment. Semin Nucl Med 2007;37:340–56. [DOI] [PubMed] [Google Scholar]
- [10].Welch Dinauer CA, Tuttle RM, Robie DK, et al. Clinical features associated with metastasis and recurrence of differentiated thyroid cancer in children, adolescents and young adults. Clin Endocrinol (Oxf) 1998;49:619–28. [DOI] [PubMed] [Google Scholar]
- [11].Jarzab B, Handkiewicz-Junak D, Wloch J. Juvenile differentiated thyroid carcinoma and the role of radioiodine in its treatment: a qualitative review. Endocr Relat Cancer 2005;12:773–803. [DOI] [PubMed] [Google Scholar]
- [12].Lazar L, Lebenthal Y, Segal K, et al. Pediatric thyroid cancer: postoperative classifications and response to initial therapy as prognostic factors. J Clin Endocrinol Metab 2016;101:1970–9. [DOI] [PubMed] [Google Scholar]
- [13].Lee YA, Jung HW, Kim HY, et al. Pediatric patients with multifocal papillary thyroid cancer have higher recurrence rates than adult patients: a retrospective analysis of a large pediatric thyroid cancer cohort over 33 years. J Clin Endocrinol Metab 2015;100:1619–29. [DOI] [PubMed] [Google Scholar]
- [14].Newman KD, Black T, Heller G, et al. Differentiated thyroid cancer: determinants of disease progression in patients <21 years of age at diagnosis: a report from the Surgical Discipline Committee of the Children's Cancer Group. Ann Surg 1998;227:533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Alessandri AJ, Goddard KJ, Blair GK, et al. Age is the major determinant of recurrence in pediatric differentiated thyroid carcinoma. Med Pediatr Oncol 2000;35:41–6. [DOI] [PubMed] [Google Scholar]
- [16].McGregor LM, Rosoff PM. Follicle-derived thyroid cancer in young people: the Duke experience. Pediatr Hematol Oncol 2001;18:89–100. [DOI] [PubMed] [Google Scholar]
- [17].Lazar L, Lebenthal Y, Steinmetz A, et al. Differentiated thyroid carcinoma in pediatric patients: comparison of presentation and course between pre-pubertal children and adolescents. J Pediatr 2009;154:708–14. [DOI] [PubMed] [Google Scholar]
- [18].Hay ID, Gonzalez-Losada T, Reinalda MS, et al. Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J Surg 2010;34:1192–202. [DOI] [PubMed] [Google Scholar]
- [19].Brown AP, Chen J, Hitchcock YJ, et al. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab 2008;93:504–15. [DOI] [PubMed] [Google Scholar]
- [20].Rubino C, de Vathaire F, Dottorini ME, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer 2003;89:1638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marti JL, Jain KS, Morris LG. Increased risk of second primary malignancy in pediatric and young adult patients treated with radioactive iodine for differentiated thyroid cancer. Thyroid 2015;25:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tuttle RM, Leboeuf R, Robbins RJ, et al. Empiric radioactive iodine dosing regimens frequently exceed maximum tolerated activity levels in elderly patients with thyroid cancer. J Nucl Med 2006;47:1587–91. [PubMed] [Google Scholar]
- [23].Lassmann M, Hanscheid H, Chiesa C, et al. EANM Dosimetry Committee series on standard operational procedures for pre-therapeutic dosimetry I: blood and bone marrow dosimetry in differentiated thyroid cancer therapy. Eur J Nucl Med Mol Imaging 2008;35:1405–12. [DOI] [PubMed] [Google Scholar]
- [24].Luster M, Lassmann M, Freudenberg LS, et al. Thyroid cancer in childhood: management strategy, including dosimetry and long-term results. Hormones (Athens) 2007;6:269–78. [DOI] [PubMed] [Google Scholar]
- [25].Lassmann M, Hanscheid H, Verburg FA, et al. The use of dosimetry in the treatment of differentiated thyroid cancer. Q J Nucl Med Mol Imaging 2011;55:107–15. [PubMed] [Google Scholar]
- [26].Maxon HR, 3rd, Englaro EE, Thomas SR, et al. Radioiodine-131 therapy for well-differentiated thyroid cancer--a quantitative radiation dosimetric approach: outcome and validation in 85 patients. J Nucl Med 1992;33:1132–6. [PubMed] [Google Scholar]
- [27].Albano D, Bertagna F, Panarotto MB, et al. Early and late adverse effects of radioiodine for pediatric differentiated thyroid cancer. Pediatr Blood Cancer 2017;64:1–7. [DOI] [PubMed] [Google Scholar]
- [28].Francis GL, Waguespack SG, Bauer AJ, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2015;25:716–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liang Y, Mi J. Pubertal hypertension is a strong predictor for the risk of adult hypertension. Biomed Environ Sci 2011;24:459–66. [DOI] [PubMed] [Google Scholar]
- [30]. Health. RGoCSPFa. Report on the Physical Fitness and Health Surveillance of Chinese School Students, 1991. [Google Scholar]
- [31].Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2018;68:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE v5.0). 2017;Washington, DC: National Cancer Institute, Avaiable from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed October 10, 2019. [Google Scholar]
- [33].Verburg FA, Hanscheid H, Biko J, et al. Dosimetry-guided high-activity (131)I therapy in patients with advanced differentiated thyroid carcinoma: initial experience. Eur J Nucl Med Mol Imaging 2010;37:896–903. [DOI] [PubMed] [Google Scholar]
- [34].Duskin-Bitan H, Leibner A, Amitai O, et al. Bone-marrow suppression in elderly patients following empiric radioiodine therapy: real-life data. Thyroid 2019;29:683–91. [DOI] [PubMed] [Google Scholar]
- [35].Prinsen HT, Klein Hesselink EN, Brouwers AH, et al. Bone Marrow Function After (131)I therapy in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 2015;100:3911–7. [DOI] [PubMed] [Google Scholar]
- [36].Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


