Abstract
Background
Congenital cytomegalovirus (CMV) infection is the most common non-genetic cause of sensorineural hearing loss (SHNL) in children. Only about 10% to 15% of children with congenital CMV are symptomatic, and most are not diagnosed at birth. About 7% to 15% of clinically asymptomatic patients may develop later complications, including SNHL, which is the most common sequela in clinically asymptomatic patients. In this study, hearing status was investigated in children with confirmed CMV infection and neonatal hearing screening (NHS) histories were reviewed to explore hearing loss caused by CMV.
Methods
The medical records of 58 children who were diagnosed with confirmed CMV infection were reviewed for clinical symptoms and signs of CMV infection. Hearing status was evaluated with age-appropriate audiological test batteries.
Results
A total of 58 children (M:F = 32:26 patients; age at study: mean, 5.62 years, range, 1-10 years) were diagnosed serologically with CMV infection (14 patients, 21.1%), or diagnosed via PCR of serum (5, 7.9%) and/or PCR from urine (19, 26.8%). Hearing loss was confirmed in 11 children (19.0%), being bilateral in 6 (54.5%), and unilateral in 5 (45.5%). Note that 7 of 17 ears with hearing loss passed NHS and were diagnosed only after re-evaluation when CMV infection was identified.
Conclusion
Hearing loss is a serious complication of CMV infection in children. Our results highlight the importance of timely audiological evaluation in children with clinically symptomatic CMV infection even if they pass NHS.
Keywords: Hearing Loss, Cytomegalovirus, Newborn Screening
Graphical Abstract

INTRODUCTION
Congenital cytomegalovirus (CMV) infection is the most common intrauterine infection in humans. The average prevalence at birth has been estimated as about 0.7%.1,2 CMV infection in utero causes children to be born with or develop many abnormalities, including petechiae, pneumonitis, hepatosplenomegaly, microcephaly, and chorioretinitis.3 In addition, many patients with congenital CMV suffer from neurological deficits, including hearing loss and vision loss, and developmental delays. At birth, only a small portion (10%–15%) of children with congenital CMV are symptomatic, and the majority are asymptomatic. The prevalence of hearing loss is 30% to 65% in symptomatic neonates and 7%–15% in asymptomatic neonates.4,5,6,7 Congenital CMV infection is the most common non-genetic cause of congenital sensorineural hearing loss (SNHL).8 Timely diagnosis and intervention are essential for children with SNHL to prevent delayed language development; thus, universal newborn hearing screening (NHS) programs have been implemented in many countries (including Korea) to identify children at high risk for congenital SNHL. However, a significant portion of children with moderate or severe bilateral hearing loss may remain undiagnosed.9 In addition, children with progressive or fluctuating hearing loss may pass the NHS performed shortly after birth. Hearing loss associated with CMV infection is known to be primarily SNHL, although conductive and mixed-type hearing loss may also be present.10 The presentation of hearing loss in those with CMV infection varies; loss may be mild to profound, and the hearing threshold may change over time.11 Hearing loss may be present at birth or develop later; fluctuation/progression of hearing loss has been reported.12,13,14 As hearing loss may not be evident at birth, children with CMV infection may pass NHS. Thus, long-term follow-up of hearing is essential in those with confirmed CMV infection.6,15,16
The incidence of congenital CMV infection depends on the population CMV seroprevalence.8 In Korea, the maternal seroprevalence of CMV IgG may be as high as 98.1%, which indicates that risk for congenital CMV infection may be of concern.17 However, little Korean data are available. One study reported an incidence of congenital CMV infection of 1.2% in a small population of Korean children.18 Another study found that CMV was a significant cause of SNHL in a selected population of neonates and infants.19 Although recent studies support the suggestion that additional targeted or universal CMV screening (in addition to NHS) would improve the detection of children at risk for hearing loss caused by CMV infection,20,21 such screening has yet been widely implemented in Korea. One recent study reported that targeted CMV screening in infants who did not pass the NHS were diagnosed with congenital CMV infection.22 Here, we performed a retrospective review of children with confirmed CMV infection, including congenital CMV infection, to describe their hearing status and describe their NHS results to provide basic information relevant to future implementation of CMV screening programs.
METHODS
We retrospectively evaluated the medical records of 58 children in whom CMV infection was confirmed before the age of 1 year and had undergone diagnostic audiological evaluations between March 2008 and December 2018 at Gangnam Severance Hospital, Yonsei University College of Medicine. The CMV infection was diagnosed using real-time PCR assay of either serum or urine specimen for viral DNA in all infants. Clinical confirmation of congenital CMV infection was made when CMV infection was confirmed during the first 2 weeks of life. All perinatal histories were reviewed for clinical symptoms and signs of CMV infection. Our hospital utilizes a two-staged NHS program using automated auditory brainstem response (AABR), but CMV testing is not routinely performed. AABR was performed using 35 dB HL click stimuli with an ALGO III AABR system (Natus Medical Inc., San Carlos, CA, USA). Infants who did not pass both steps of screening, in either or both ears, were referred for diagnostic audiological evaluation in our ENT department. Usual schedule for diagnostic audiologic evaluations were at 1 month and follow-up at 3 and 6 months if needed. At the time of the study, CMV testing was not routinely recommended for infants who did not pass the NHS. When infants who did not pass NHS were referred, we evaluated hearing status using age-appropriate audiological test batteries, including auditory brainstem response (ABR), auditory steady-state response (ASSR) and transiently evoked otoacoustic emission (TEOAE). All tests were performed in a double-walled soundproof booth in accordance with ANSI standard S3.1-1999 (R 2008). ABRs were evaluated using the GSI Audera® AEP system running dedicated software (Grason-Stadler, Eden Prairie, MN, USA). Disposable electrodes (Nicolet Biomedical, Madison, WI, USA) were placed on the high forehead and on the ipsilateral and contralateral mastoids. All electrodes had impedances < 2kΩ. Stimuli (50-µs clicks at 90 dB peak equivalent sound pressure level [peSPL]) were presented via the Telephonics TDH 49 headphones at a rate of 11 clicks/s, with alternating polarity. The signals were bandpass-filtered (100–1,500 Hz) and averaged over at least 8,000 repetitions. The amplitudes of waves I and V were measured from the peak to the following troughs. ASSR was recorded using GSI Audera system (Grason-Stadler, Madison, MI, USA) Electrode discs of Ag/AgCl were fixed with electrolytic paste at Cz, Fz and ear lobe. TEOAEs were recorded using ILO 92 Otodynam- ics analyzer system (Otodynamics, England, UK) and ILO V6 software. A click stimulus with 80 μs duration was presented at a repetition interval of 20 msec. TEOAEs were considered present (“pass”) if the signal-to-noise ratio was 6 dB or great in at least 3 of 5 frequency bands, and absent (“refer”) otherwise. Hearing was categorized as normal (0–25 dB) or with mild (26–40 dB), moderate (41–55 dB), moderate to severe (56–70 dB), severe (71–90 dB), or profound (≥ 91dB) hearing loss according to ISO 1964 as in previous studies.4,19 We graded hearing status using the hearing threshold of the better ear. Asymmetrical hearing loss was defined as more than 15 dB difference in hearing thresholds in both ears. Deterioration or improvement in hearing was defined as a change of ≥ 10 dB in the hearing threshold. Descriptive analyses were performed using SPSS for Windows ver. 12.0 (IBM, Chicago, IL, USA). All data are presented as means ± standard deviations.
Ethics statement
This study was approved by the Institutional Review Board (IRB) of Yonsei University College of Medicine Gangnam Severance Hospital (IRB No. 3-2019-0220). The IRB approved the conduction of this study without the informed consent from the participants because this study used anonymized retrospective EMR data.
RESULTS
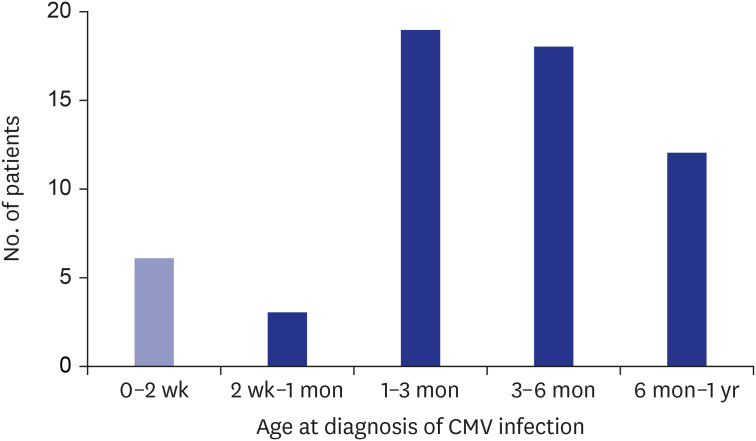
A total of 58 children (M:F = 32:26; age at study: mean, 94 months, range, 40-143 months) were included. CMV infection was diagnosed serologically (IgM, 14 patients, 21.1%) or by PCR of serum (5, 7.9%) and/or urine (19, 26.8%). CMV infection was diagnosed before 6 months of age in most patients (46 patients, 79.3%) (Fig. 1). Among the 18 patients (31.0%) who were diagnosed before 1 month of age, 6 patients were diagnosed within the first 2 weeks as congenital CMV infection. CMV screening is not yet part of the universal neonatal screening; the reasons for CMV screening include maternal CMV seroconversion or abnormalities evident on physical examinations or neurosonography. The clinical manifestation of CMV infection were reviewed; 16 patients (27.6%) were asymptomatic, and 35 (60.3%) were symptomatic, and medical records were inadequate in 5 patients (8.6%) (Table 1). Clinical symptoms and signs included hepatitis (62.9%), hyperbilirubinemia (28.6%), pneumonia (14.3%), and hepatic splenomegaly (5.7%), and chorioretinitis (2.9%).
Fig. 1. Age at confirmation of CMV infection (n = 58). The figure shows distribution of patients who were confirmed of CMV infection at each age group, including 6 patients with congenital CMV infection confirmed within the first 2 weeks after birth.
CMV = cytomegalovirus.
Table 1. Clinical manifestations in 58 children with confirmed congenital cytomegalovirus infection.
| Group | Symptoms | Values |
|---|---|---|
| Symptomatic (n = 35, 60.3%) | Hepatitis | 22 (62.9) |
| Hyperbilirubinemia | 10 (28.6) | |
| Pneumonia | 5 (14.3) | |
| Hepatosplenomegaly | 2 (5.7) | |
| Chorioretinitis | 1 (2.9) | |
| Asymptomatic (n = 16, 27.6%) | ||
| Unclear (n = 7, 12.1%) |
Values are presented as number (%).
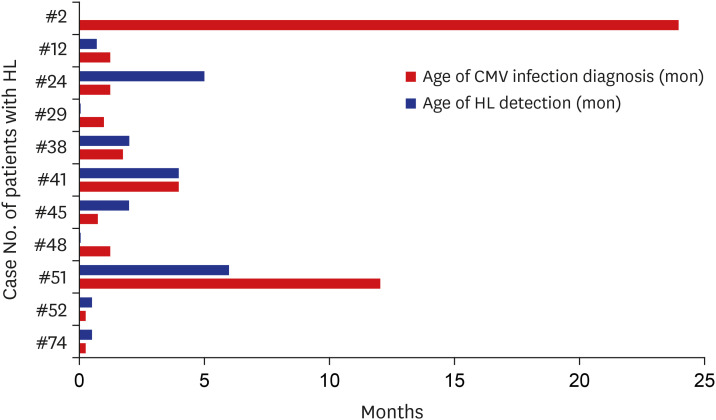
Children were referred for diagnostic audiological evaluation when CMV infection was confirmed, but the tests were performed later in some children (when the general condition stabilized). Hearing thresholds were estimated from click-evoked ABR and/or ASSR measurements. The mean age at the diagnosis of hearing loss was 2.27 ± 3.52 months (range, 0.25–12 months) (Fig. 2). Six patients were diagnosed with hearing loss before CMV infection was confirmed. Five were diagnosed with CMV infection and repeat audiological evaluations then confirmed hearing loss.
Fig. 2. Age at detection of HL and diagnosis of CMV infection (n = 11). Most patients were diagnosed with CMV infection before hearing loss was confirmed.
HL = hearing loss, CMV = cytomegalovirus.
Hearing loss was diagnosed in 11 patients (19.0%), being bilateral in 6 (54.5%) (Table 2). Hearing loss was mild in one patient, moderate in two, moderate to severe in one, and severe in two. The NHS results of all children were reviewed (Table 3). Seven of the 17 ears (41.2%) with confirmed hearing loss and CMV infection had passed NHS. The hearing status of these ears varied, ranging from normal to profound in terms of hearing loss. The clinical characteristics and hearing status of the 11 patients are listed in Table 4. Seven patients (63.6%) were admitted to NICU for 28.4 ± 25.7 days (range, 13 to 90 days). Brain imaging studies showed abnormalities in 5 patients (45.5%), but abnormalities in the inner ear structures were not identified. Follow-up audiologic tests were performed in 8 patients; most evidenced no change in hearing status. One patient exhibited fluctuation of hearing in both ears; another showed improved hearing in one ear.
Table 2. Hearing status of children with HL and confirmed congenital cytomegalovirus infection (n = 11).
| Patients (n = 11) | Values | |
|---|---|---|
| Unilateral HL | 5 (45.5) | |
| Bilateral HL | 6 (54.5) | |
| Hearing status of the better ear | ||
| Normal | 5 (50.0) | |
| Mild HL | 1 (9.1) | |
| Moderate HL | 2 (18.2) | |
| Moderate to severe HL | 1 (9.1) | |
| Severe HL | 2 (18.2) | |
| Profound HL | 0 (0.0) | |
Values are presented as number (%).
HL = hearing loss.
Table 3. Hearing status and NHS results of the ears (n = 22) of children with HL.
| Hearing status | Total | Passed NHS | Failed NHS | |
|---|---|---|---|---|
| Ears (n = 22) | ||||
| Normal | 5 (22.7) | 3 (60.0) | 2 (40.0) | |
| HL | 17 (77.3) | 7 (41.2) | 10 (58.8) | |
| Ears with HL (n = 17) | ||||
| Mild HL | 4 (23.5) | 2 (50.0) | 2 (50.0) | |
| Moderate HL | 2 (11.8) | 0 (0.0) | 2 (100.0) | |
| Moderate to severe HL | 5 (29.4) | 1 (20.0) | 4 (80.0) | |
| Severe HL | 1 (5.9) | 1 (100.0) | 0 (0.0) | |
| Profound HL | 5 (29.4) | 3 (60.0) | 2 (40.0) | |
Values are presented as number (%).
NHS = neonatal hearing screening, HL = hearing loss.
Table 4. Clinical characteristics of children with confirmed congenital cytomegalovirus infection and HL (n = 11).
| Patient No. | Sex | Laterality | Initial hearing threshold, right/left, dB | Progress of HL | Follow-up period, mon | NHS, right/left | NICU stay, day | Neuroimaging |
|---|---|---|---|---|---|---|---|---|
| #2 | F | Bilateral, symmetrical | NR/80 | - | - | Pass/Pass | 90 | Brain CT: wnl |
| #12 | F | Bilateral, symmetrical | 60/50 | Stable | 14 | Refer/Refer | 20 | Brain CT: wnl |
| #24 | M | Unilateral | 50/40 | Fluctuating (range, 30–50 dB) | 6 | Pass/Pass | - | Brain CT: wnl |
| #29 | F | Bilateral, asymmetrical | NR/50 | Stable | 3 | Refer/Refer | 16 | Brain MRI: polymicrogyria, cortical dysplasia |
| #38 | F | Bilateral, symmetrical | 70/60 | Stable | 2 | Refer/Refer | - | Brain MRI: CSF space widening |
| #41 | F | Bilateral, asymmetrical | 40/60 | - | - | Pass/Pass | - | Brain MRI: microphthalmia, CSF space widening |
| #45 | M | Unilateral | 80/25 | Improving (40/25 after 8 mon) | 8 | Refer/Refer | 28 | Neurosonography: wnl |
| #48 | M | Unilateral | 40/25 | Stable | 3 | Refer/Refer | 11 | Brain CT: wnl |
| #51 | M | Unilateral | 25/NR | Stable | 8 | Pass/Pass | - | Neurosonography: wnl |
| #52 | F | Unilateral | 20/NR | Stable | 14 | Pass/Pass | 21 | Brain CT: wnl |
| #74 | F | Bilateral, asymmetrical | NR/50 | - | - | Refer/Refer | 13 | Brain MRI: polymicrogyria |
HL = hearing loss, NHS = neonatal hearing screening, NICU = neonatal intensive care unit, CT = computed tomography, MRI = magnetic resonance imaging.
DISCUSSION
We investigated how children with hearing loss related to CMV infection were initially diagnosed and to highlight here the importance of audiological evaluation of such children. In our hospital, all neonates undergo NHS, but CMV screening tests are not routinely performed. Children are recommended for such tests only if there is a clinical suspicion of perinatal CMV infection or when maternal CMV infection is clinically suspected or diagnosed. Our pediatricians and otologists in our hospital agree that formal, diagnostic audiological evaluation is appropriate if CMV infection is confirmed via PCR of urine or serum samples, even if children initially pass NHS. Overall, 11/58 patients (19.0%) with confirmed CMV infection were diagnosed with hearing loss; this incidence is less than that in a study of Korean children (hearing loss was present in 33.3% of those with congenital CMV infection in that study).19 Several factors may explain this difference. Our patients had confirmed with CMV infection within 1 year of birth, but only 6 patients were diagnosed with congenital CMV infection, which must be confirmed within the first 2 weeks of life. It is noteworthy that 5 of 6 patients (83.3%) with congenital CMV infection were also diagnosed with hearing loss, while hearing loss was less frequent in patients later diagnosed with CMV infection (6/52 patients, 11.5%). In contrast, Kim et al. investigated children diagnosed with congenital CMV. It is not possible to directly compare the frequency of hearing loss in that study to that in our present work. In addition, in our work, initial clinical suspicion of CMV infection was followed by a delay prior to laboratory confirmation of CMV infection. This was considered inappropriate, and we have revised (improved) our CMV testing protocol as a result. Kim et al.'s study19 included three distinct groups of children: those diagnosed clinically with congenital CMV, those recruited from neonatal intensive care units (NICU), and SNHL patients with confirmed congenital CMV infection who underwent cochlear implantation. As Kim et al.22 noted, NICU patients more commonly arrive preterm than other infants, and their prevalence of hearing loss may not reflect that of the general infant population. Our retrospective study enrolled children with confirmed CMV infection; these are also not representative of the general population. However, both studies support the suggestion that it is important to evaluate the hearing of children with CMV infection. Another study reported that when the newborns who did not pass NHS were screened for CMV, 1.5% of them were positive, and 0.8% of the newborns who did not pass NHS were diagnosed with asymptomatic congenital CMV infection, highlighting the potential advantage of timely CMV screening. With increased attention to early identification and treatment of congenital CMV infection, two approaches for CMV screening in newborns have been proposed. While the diagnostic benefits of universal screening of congenital CMV infection in all newborns are undebatable, targeted CMV screening of newborns who do not pass NHS has been adopted in some hospitals and locations.5,22,23 However, targeted screening of CMV inevitably will miss cases of delayed onset hearing loss in patients with congenital CMV infection. A recent study suggested that addition of genetic and CMV screening to preexisting NHS programs would help identify newborns with increased risk of hearing loss due to congenital CMV infection.21 Our data also shows that passing the NHS does not necessarily rule out hearing loss in CMV infection, especially congenital CMV infected infants, supporting the benefit of universal CMV screening in all newborns.
Of the various causes of congenital SNHL, CMV infection is worthy of clinical attention because antiviral medications are available. We could not determine the effect of treatment of CMV infection on hearing loss because follow-up hearing evaluation was not performed in the two patients with ganciclovir treatment (case no. #41, 74). In one study, treatment of symptomatic congenital CMV children with ganciclovir prevented hearing loss (compared to a control group).24 Oral valganciclovir improved hearing and language outcomes.25 In another study that treated symptomatic congenital CMV infection with valganciclovir for 6 months, hearing was improved in the long term compared to a group with 6 weeks of therapy.26 However, a recent study of 16 patients treated with valganciclovir revealed a measurable, but not a statistically significant, decline in hearing.27 Thus, hearing outcomes vary among studies; and the antiviral data were all obtained from patients with symptomatic CMV infection. Further long-term studies featuring comprehensive audiological evaluation and long-term follow-ups are needed to better understand the benefits and risks of antiviral therapies in terms of hearing loss. However, it is clear that patients with CMV and SNHL require particular attention. The universal NHS program is available through the Korean National Health Insurance Service in Korea and plays an invaluable role in terms of early identification of, and intervention for, hearing loss. However, the NHS protocol may not identify children with progressive hearing loss (who pass initial NHS testing but later develop hearing loss), or children with fluctuating hearing thresholds (which is possible in hearing loss related to CMV infection). We found that passing the NHS did not necessarily guarantee later normal hearing, particular for patients with confirmed CMV infection. Clinicians treating such children should be aware that, even if the children have passed NHS, a diagnostic audiologic evaluation is appropriate. Regular follow-up hearing tests are recommended to ensure that progressive/fluctuating hearing loss is detected. In Korea, routine hearing screening is universally available in the first year of elementary school. Prior to that time, we recommend yearly hearing follow-up in the hospital.
This descriptive study is an initial attempt to emphasize the importance of audiological evaluation of children with CMV infection and the need for interspecialty consultation to identify hearing loss in a timely manner. A strength of our study is that we performed diagnostic audiological tests; we did not rely on the hearing screen to identify hearing loss. Limitations of the study include the retrospective nature of the work and the relatively small number of patients enrolled; a bias toward including more severely affected patients may have been in play. In addition, the timing of audiological evaluations was varied, and only a few patients underwent such evaluations. Another limitation involves the lack of neurodevelopmental data. Apart from the risk of delayed language development attributable to hearing loss, neurodevelopment should be regularly checked, in particular in symptomatic patients. Further studies are needed to define the progression of hearing loss in patients with CMV infection and the possible therapeutic utility of antivirals in terms of long-term improvement or stabilization of hearing. Regular follow-up of audiological evaluation is essential for children with confirmed CMV infection.
Hearing loss is an important manifestation of both symptomatic and asymptomatic CMV infection. Our data are in line with reports that indicate that hearing loss is associated with confirmed CMV infection and show that the NHS program inadequately identifies such loss. Our results support the suggestion that more comprehensive screening for CMV-related hearing loss is required in Korea.
Footnotes
Funding: This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (grant No. NRF-2019R1F1A1062836) and by a faculty research grant from Yonsei University College of Medicine (No. 6-2018-0107).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Son EJ.
- Data curation: Kim JH, Nam GS.
- Formal analysis: Roh KJ.
- Methodology: Kim JH, Son EJ.
- Writing - original draft: Kim JH, Roh KJ.
- Writing - review & editing: Roh KJ, Nam GS, Son EJ.
References
- 1.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 2.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 3.Mestas E. Congenital cytomegalovirus. Adv Neonatal Care. 2016;16(1):60–65. doi: 10.1097/ANC.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 4.Fowler KB, Dahle AJ, Boppana SB, Pass RF. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135(1):60–64. doi: 10.1016/s0022-3476(99)70328-8. [DOI] [PubMed] [Google Scholar]
- 5.Fowler KB, McCollister FP, Sabo DL, Shoup AG, Owen KE, Woodruff JL, et al. A targeted approach for congenital cytomegalovirus screening within newborn hearing screening. Pediatrics. 2017;139(2):e20162128. doi: 10.1542/peds.2016-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goderis J, Keymeulen A, Smets K, Van Hoecke H, De Leenheer E, Boudewyns A, et al. Hearing in children with congenital cytomegalovirus infection: results of a longitudinal study. J Pediatr. 2016;172:110–115.e2. doi: 10.1016/j.jpeds.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Riga M, Korres G, Chouridis P, Naxakis S, Danielides V. Congenital cytomegalovirus infection inducing non-congenital sensorineural hearing loss during childhood; a systematic review. Int J Pediatr Otorhinolaryngol. 2018;115:156–164. doi: 10.1016/j.ijporl.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26(1):86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkin PM, Baldwin M. Identifying deafness in early childhood: requirements after the newborn hearing screen. Arch Dis Child. 2011;96(1):62–66. doi: 10.1136/adc.2010.185819. [DOI] [PubMed] [Google Scholar]
- 10.Dobbie AM. Evaluation and management of cytomegalovirus-associated congenital hearing loss. Curr Opin Otolaryngol Head Neck Surg. 2017;25(5):390–395. doi: 10.1097/MOO.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 11.Foulon I, Naessens A, Faron G, Foulon W, Jansen AC, Gordts F. Hearing thresholds in children with a congenital CMV infection: a prospective study. Int J Pediatr Otorhinolaryngol. 2012;76(5):712–717. doi: 10.1016/j.ijporl.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Williamson WD, Demmler GJ, Percy AK, Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics. 1992;90(6):862–866. [PubMed] [Google Scholar]
- 13.Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol. 2000;11(5):283–290. [PubMed] [Google Scholar]
- 14.Madden C, Wiley S, Schleiss M, Benton C, Meinzen-Derr J, Greinwald J, et al. Audiometric, clinical and educational outcomes in a pediatric symptomatic congenital cytomegalovirus (CMV) population with sensorineural hearing loss. Int J Pediatr Otorhinolaryngol. 2005;69(9):1191–1198. doi: 10.1016/j.ijporl.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Sabroske E, Svoboda MD, Ng YT. Passing the newborn hearing screen does not always exclude acquired hearing loss due to congenital infection. Pediatr Neurol. 2018;83:60–61. doi: 10.1016/j.pediatrneurol.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Haesen S, Shaw D. Clinical characteristics, audiological and neurodevelopmental outcomes of newborns with congenital cytomegalovirus infection. Swiss Med Wkly. 2018;148:w14628. doi: 10.4414/smw.2018.14627. [DOI] [PubMed] [Google Scholar]
- 17.Seo S, Cho Y, Park J. Serologic screening of pregnant Korean women for primary human cytomegalovirus infection using IgG avidity test. Korean J Lab Med. 2009;29(6):557–562. doi: 10.3343/kjlm.2009.29.6.557. [DOI] [PubMed] [Google Scholar]
- 18.Sohn YM, Park KI, Lee C, Han DG, Lee WY. Congenital cytomegalovirus infection in Korean population with very high prevalence of maternal immunity. J Korean Med Sci. 1992;7(1):47–51. doi: 10.3346/jkms.1992.7.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim BJ, Han JJ, Shin SH, Kim HS, Yang HR, Choi EH, et al. Characterization of detailed audiological features of cytomegalovirus infection: a composite cohort study from groups with distinct demographics. BioMed Res Int. 2018;2018:7087586. doi: 10.1155/2018/7087586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronchi A, Shimamura M, Malhotra PS, Sánchez PJ. Encouraging postnatal cytomegalovirus (CMV) screening: the time is NOW for universal screening! Expert Rev Anti Infect Ther. 2017;15(5):417–419. doi: 10.1080/14787210.2017.1303377. [DOI] [PubMed] [Google Scholar]
- 21.Lu CY, Tsao PN, Ke YY, Lin YH, Lin YH, Hung CC, et al. Concurrent hearing, genetic, and cytomegalovirus screening in newborns, Taiwan. J Pediatr. 2018;199:144–150.e1. doi: 10.1016/j.jpeds.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Lee YK, Ko SY, Shin SM. Diagnostic clues for congenital cytomegalovirus infection: association with newborn hearing screening tests. Neonatal Med. 2019;26(2):96–101. [Google Scholar]
- 23.Vancor E, Shapiro ED, Loyal J. Results of a targeted screening program for congenital cytomegalovirus infection in infants who fail newborn hearing screening. J Pediatric Infect Dis Soc. 2019;8(1):55–59. doi: 10.1093/jpids/pix105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimberlin DW, Lin CY, Sánchez PJ, Demmler GJ, Dankner W, Shelton M, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143(1):16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- 25.Schleiss MR. Congenital cytomegalovirus infection: update on management strategies. Curr Treat Options Neurol. 2008;10(3):186–192. doi: 10.1007/s11940-008-0020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimberlin DW, Jester PM, Sánchez PJ, Ahmed A, Arav-Boger R, Michaels MG, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372(10):933–943. doi: 10.1056/NEJMoa1404599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCrary H, Sheng X, Greene T, Park A. Long-term hearing outcomes of children with symptomatic congenital CMV treated with valganciclovir. Int J Pediatr Otorhinolaryngol. 2019;118:124–127. doi: 10.1016/j.ijporl.2018.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]