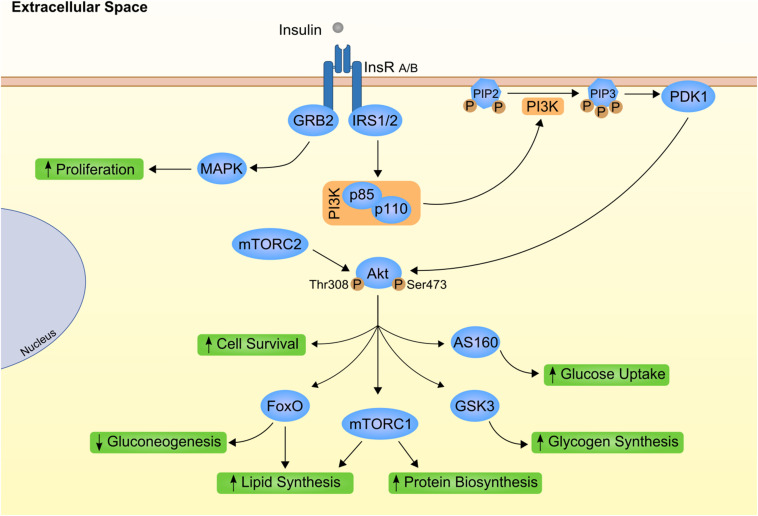
FIGURE 1.
Canonical insulin signaling cascade. Insulin (gray circle) binds to its transmembrane kinase receptor at cell membrane and triggers insulin signaling cascade. Insulin receptor present two isoforms with distinct affinity for insulin already associated to differential insulin internalization (INSR A and INSR B; Calzi et al., 1997). Binding of insulin auto-phosphorylates INSR at Tyr-960. Further recruitment and phosphorylation of insulin receptor substrate (IRS) 1 and 2 will mostly result in subsequent activation of phosphoinositide-dependent kinase 1 (PDK1) through phosphoinositide-3 kinase (PI3K; Yamada et al., 2002). PDK1 is responsible for propagation of insulin signal to one of the most important downstream effectors, Akt. Importantly, Akt has two distinct phosphorylation sites, Thr308 activated by PDK1 (Alessi et al., 1997) and Ser473 phosphorylated by mammalian target of rapamycin complex (mTORC) 2 protein (Bayascas and Alessi, 2005). Finally, fully activated Akt can interact with different proteins, eliciting different effects as stimulation of glucose uptake and glycogen synthesis by AS160 and glycogen synthase kinase 3 (GSK3), respectively (Ng et al., 2008). On the other hand, INRS activation also promote growth factor receptor-bound protein 2 (GRB2) interaction with Shc proteins and activation of mitogen activated protein kinases (MAPK; Skolnik et al., 1993; Xu et al., 2006). This as part of the insulin-mediated proliferative stimuli. PIP2, phosphatidylinositol 4,5 biphosphate; PIP3, phosphatidylinositol 3,4,5 triphosphate.