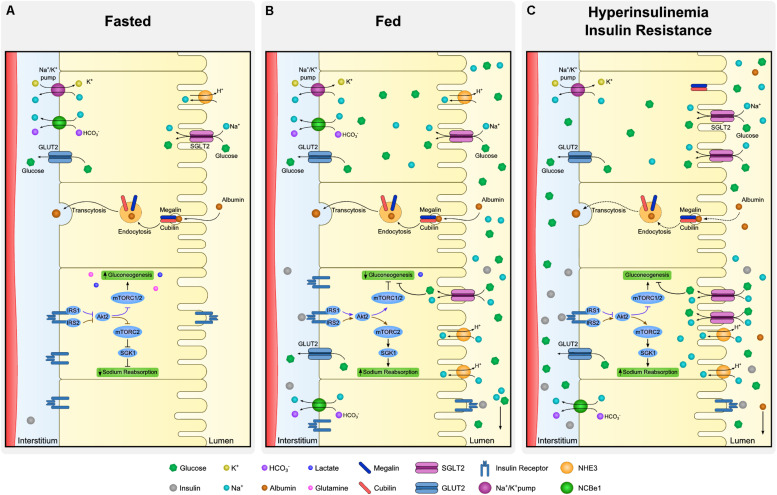
FIGURE 4.
Dynamics of proximal tubule cells at fasting, fed and insulin resistant states. Proximal tubule cells are subjected to distinct microenvironments (lumen and interstitium) and the regulation of absorption and reabsorption of molecules is complex. Although all the described processes occur in every cell of the proximal tubule simultaneously, each specific process is illustrated in a different cell. At fasting (A), low levels of insulin allow expression of gluconeogenic enzymes whereas sodium reabsorption is downregulated. Expression of glucose transporter 2 (GLUT2) at basolateral membrane is mostly associated to glucose output and not to its uptake. Moreover, albumin absorption is performed by megalin and cubilin at luminal membrane and transcytosis allow albumin to be rerouted back to the organism. At fed state (B), increased availability of insulin and glucose promote drastic changes in proximal tubule dynamics. In the case of insulin, luminal uptake is mostly associated to degradation and basolateral to signaling activation. Insulin receptor (INSR) activation downregulates gluconeogenesis and increases sodium reabsorption by different proteins as type 3 Na-H exchanger (NHE-3) and sodium-glucose transport protein 2 (SGLT2). Together with sodium, SGLT2 also co-transport glucose from the lumen. Finally, hyperinsulinemia is linked to perturbations of proximal tubule cells in many aspects (C). As in many other organs, insulin signaling desensitization is associated to inefficient inhibition of gluconeogenesis contributing to maintenance of increased levels of glucose. Derangements at podocyte level increases filtration of albumin and overloads luminal capacity of reabsorption. Such impairment in albumin reabsorption culminates with albuminuria, frequent observed in hyperinsulinemic states.