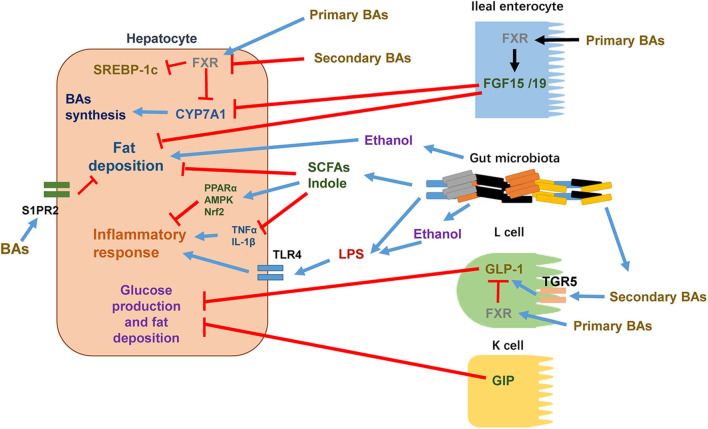
Figure 1.
The crosstalk between intestine and liver in the pathophysiology of NAFLD. Certain intestine hormones, e.g., GLP-1 and GIP, reduce hepatic glucose production and fat accumulation. In L cells, secondary BAs stimulate GLP-1 synthesis and release via TGR5 activation whereas primary BAs activate FXR to inhibit GLP-1 synthesis and release. Other intestine hormones, e.g., FGF15 and FGF19, decrease hepatic lipogenesis. BAs stimulate FXR in ileal enterocytes, leading to the release of FGF15/19 into circulation. After reaching to hepatocytes, FGF15/19 suppresses BA synthesis through inhibiting CYP7A1 expression. Increased gut permeability, altered composition of gut microbiota, and elevated levels of gut microbiota metabolites such as ethanol are shown to enhance hepatocyte fat deposition and increase the flow of LPS into the circulation to promote proinflammatory responses through activating TLR4 signaling pathway in target cells. In hepatocytes, certain primary BAs acts through activating FXR to suppress the activity of SREBP-1c and thus reduces the expressions of lipogenic genes. Primary BAs also inhibit CYP7A1 expression and thus reduces BAs synthesis. Certain secondary BAs inhibit the activation of hepatic FXR. In addition to activation of FXR, BAs are shown to regulate hepatic lipid and sterol metabolism through activating S1PR2. Certain gut microbiota metabolites such as SCFAs and indole reduce hepatocyte fat deposition and proinflammatory responses via decreasing TNFα and IL-1β and/or activating PPARα, AMPK, and Nrf2.