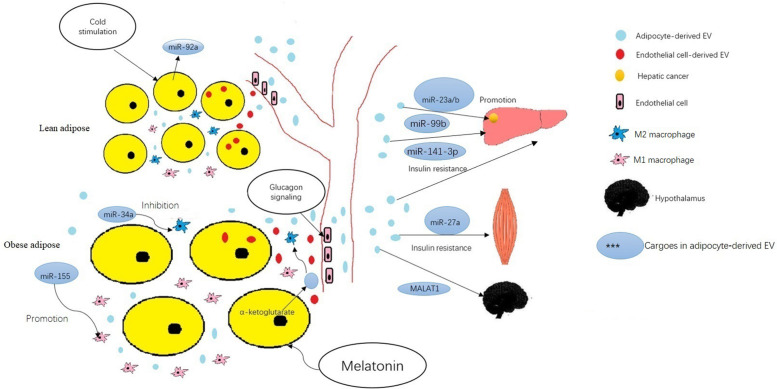
FIGURE 1.
The roles of miRNAs and other cargoes mentioned in the text contained in adipocyte-derived EVs in regulating metabolism and development of obesity-associated disease. ∗The cargoes in adipocyte-derived EVs adjust in physiological and pathological states. Cold stimulation down regulates the circulating miR-92a in EVs of adipose tissue. Adipocytes may receive EVs that contain membrane proteins from endothelial cells, then transport these proteins into adipocyte-derived EVs. Glucagon signaling enhanced endothelial cell uptake and packaging of extracellular cargos into EVs in mice fed a high-fat diet. ∗Adipose−derived EVs mediate inter−organ cross-talk. The miR-99b in adipocyte-derived EVs regulated gene expression in the liver. Adipocyte-derived EV-associated MALAT1 regulated signaling in hypothalamic POMC neurons. In obesity, miR-141-3p in adipocyte-derived EVs promoted insulin resistance in hepatocytes and miR-27a in adipocyte-derived EVs promoted insulin resistance in skeletal muscle cells. ∗Adipose−derived EVs mediate inter−organ cross-talk. Adipose−derived EVs regulate intra−organ cross-talk between adipocytes and ATM. The miR-155 in adipocyte-derived EVs modulates of M1 macrophage polarization. The miR-34a in adipocyte-derived EVs inhibits of M2 polarization. The miRNA 23a/b in adipose−derived EVs promotes hepatic cancer cell growth, migration, and development of chemoresistance. ∗Adipocyte-derived EVs act as an intermediary between hormones and cells. Melatonin administration increases levels of α−ketoglutarate in EVs secreted by adipocytes; these EVs then promote M2 macrophage activation through increased α−ketoglutarate levels in macrophages.