Figure 4.
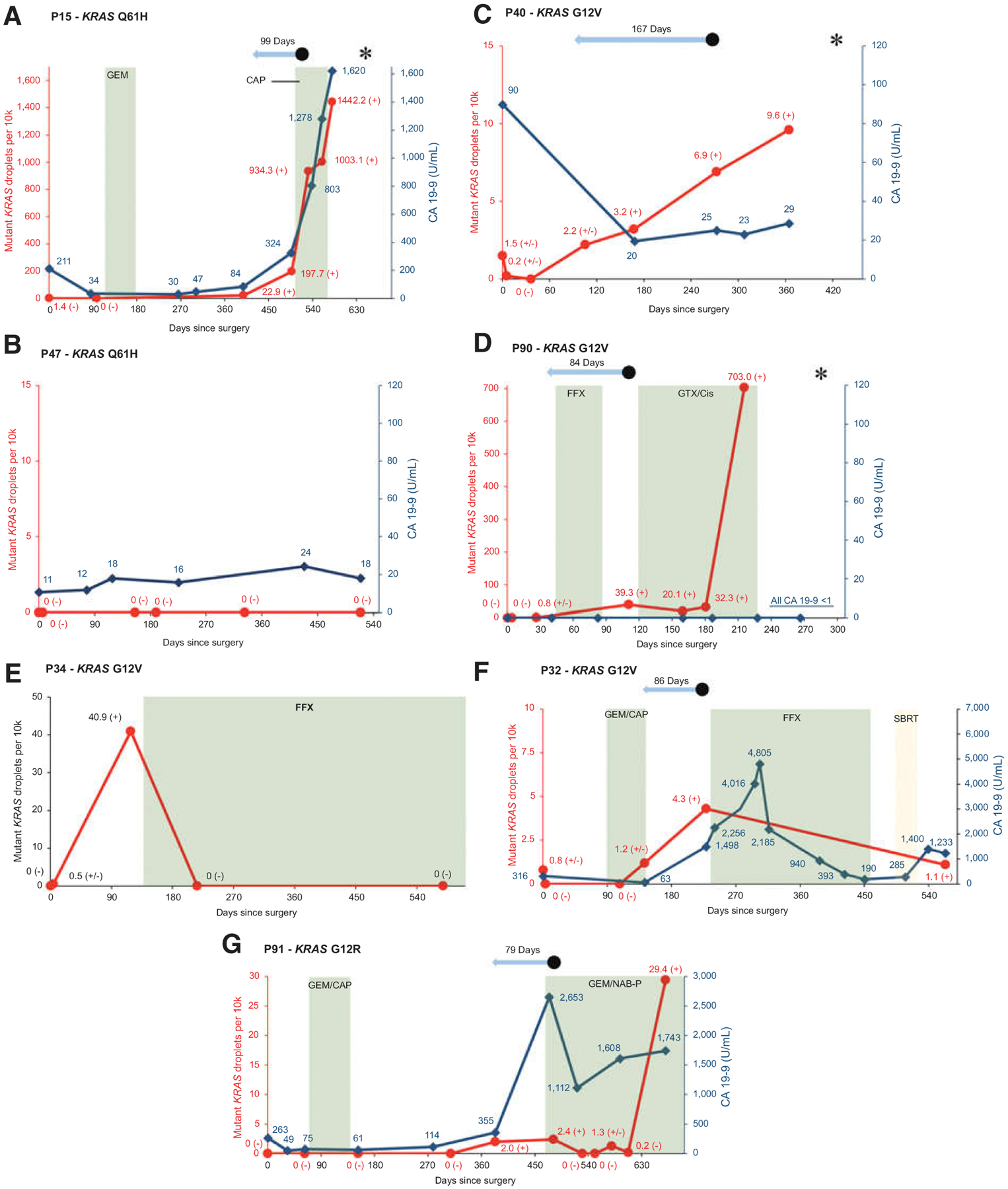
Examples of individual patient graphs showing matching rise in CA 19–9 and KRAS ctDNA in a patient with recurrence (A), similar stability in CA 19–9 and KRAS ctDNA in a patient without recurrence (B), rise in KRAS ctDNA with low and stable CA 19–9 in a patient with recurrence (C), rise in KRAS ctDNA in nonexpressor of CA 19–9 with recurrence (D), KRAS ctDNA response to treatment (E and F), and a patient in whom KRAS ctDNA might have helped clinical decision-making (G).  Red line signifies mutant KRAS droplets per 10k total droplets over time.
Red line signifies mutant KRAS droplets per 10k total droplets over time.  Dark blue line signifies CA 19–9 levels in U/mL over time.
Dark blue line signifies CA 19–9 levels in U/mL over time.  Green plane signifies chemotherapy treatment. CAP, capecitabine; FFX, FOLFIRINOX; GEM, gemcitabine; GTX/Cis, gemcitabine, docetaxel, capecitabine and cisplatin; NAB-P; nab-paclitaxel.
Green plane signifies chemotherapy treatment. CAP, capecitabine; FFX, FOLFIRINOX; GEM, gemcitabine; GTX/Cis, gemcitabine, docetaxel, capecitabine and cisplatin; NAB-P; nab-paclitaxel.  Yellow plane signifies radiotherapy. SBRT, stereotactic body radiation therapy.
Yellow plane signifies radiotherapy. SBRT, stereotactic body radiation therapy.  Light blue arrow signifies lead time of KRAS ctDNA detection relative to clinical recurrence. • signifies clinical disease recurrence; * signifies patient death; (−) signifies a negative KRAS ctDNA result; (±) signifies a borderline KRAS ctDNA result; (+) signifies a positive KRAS ctDNA result. Sample at time point 0 represents the preoperative sample.
Light blue arrow signifies lead time of KRAS ctDNA detection relative to clinical recurrence. • signifies clinical disease recurrence; * signifies patient death; (−) signifies a negative KRAS ctDNA result; (±) signifies a borderline KRAS ctDNA result; (+) signifies a positive KRAS ctDNA result. Sample at time point 0 represents the preoperative sample.
