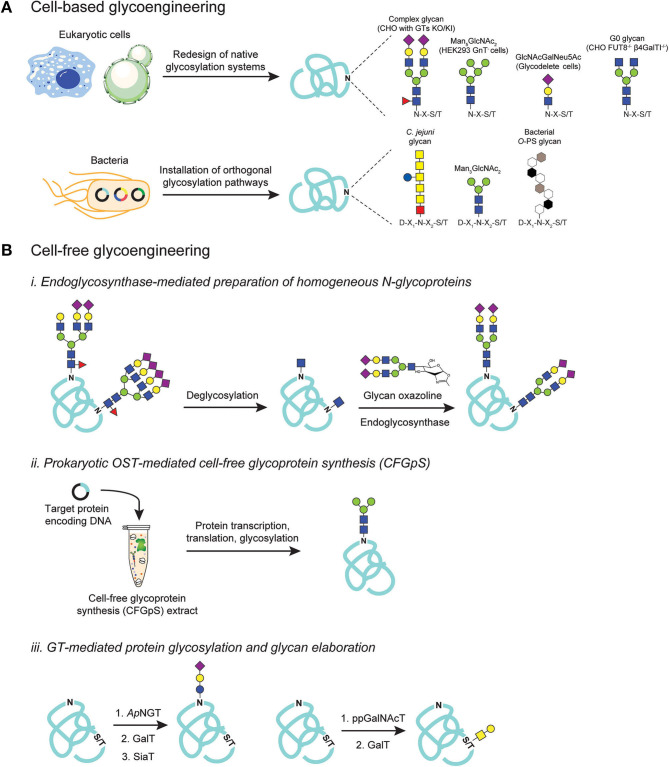
Figure 3.
Glycoengineering strategies for producing homogeneous glycoproteins. (A) Cell-based glycoengineering involves redesign of native protein glycosylation pathways in the host organism to control glycoforms. Precise genome editing strategies such as ZFNs and CRISPR/Cas9 systems have been employed to knock-out (KO) and knock-in (KI) genes to alter endogenous glycosylation networks for producing proteins bearing desirable glycoforms such as Man5GlcNAc2, GlcNAcGalNeu5Ac, G0, and complex-types with sialic acid-capped glycoforms. Alternatively, orthogonal expression of protein glycosylation pathways in E. coli can be utilized to produce proteins that are site-specifically modified with bacterial N-glycans, eukaryotic glycans such as Man3GlcNAc2, and bacterial O-polysaccharide (O-PS) antigens. (B) Cell-free glycoengineering using glycoenzymes including (i) endoglycosynthases (ENGases), (ii) prokaryotic oligosaccharyltransferases (OSTs), and (iii) glycosyltransferases (GTs). For ENGase-mediated glycosylation, glycoproteins bearing heterogenous N-glycoforms are deglycosylated using specific glycosyl hydrolases (GHs) to generate monosaccharide handle such as GlcNAc at the native glycosylation site. Pre-synthesized glycan structures containing an oxazoline functional group at the reducing end are then used as glycosyl donor in a reaction catalyzed by ENGase to remodel glycans to homogeneity. For prokaryotic OST-mediated glycosylation, cell-free extract is generated from glycoengineered E. coli such that the extract is enriched with all necessary gene transcription, protein translation, and protein glycosylation machineries. Supplementing extracts with DNA encoding target protein co-activates protein synthesis and site-specific protein glycosylation. For GT-mediated protein glycosylation, sequential glycosylation reactions are carried out, beginning with installation of an initial monosaccharide on the protein using a specific GT such as ApNGT that installs Glc on asparagine residues and ppGalNAcT that modifies serines or threonines with GalNAc. The monosaccharide primer can then be extended, directly on glycoprotein, by a series of specific GTs such as GalT and SiaT to generate a final glycoform.