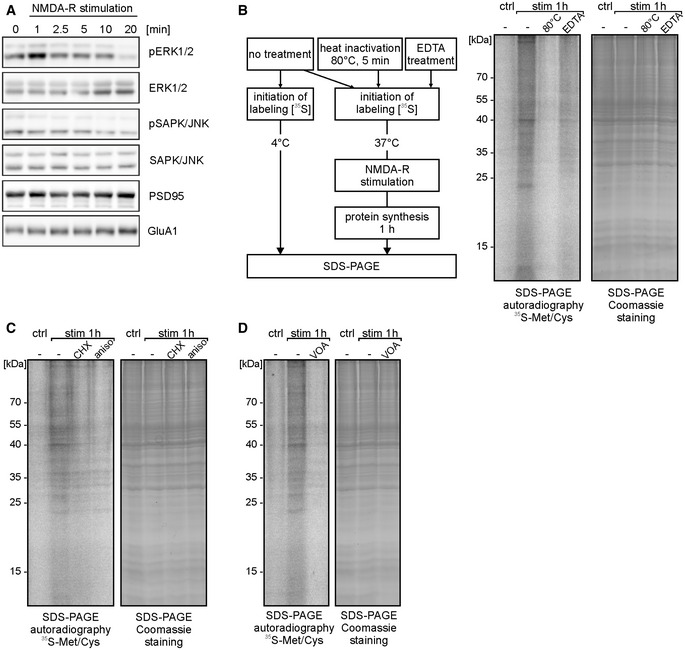
Figure EV1. Related to Fig 1. NMDA‐R stimulation of synaptoneurosomes leads to transient ERK1/2 phosphorylation and induces de novo protein synthesis.
-
AWestern blot on synaptoneurosomes verifying activation of selected kinases in response to the stimulation (0‐20 min). Chosen NMDA‐R stimulation protocol leads to transient phosphorylation of extracellular signal‐regulated protein kinases 1 and 2 (ERK1/2). In contrast, stress‐activated protein kinase/c‐Jun NH2‐terminal kinase (SAPK/JNK) is not phosphorylated upon treatment. Antibodies recognizing total ERK1/2 and total SAPK/JNK were used to ensure equal protein levels of analyzed kinases. Anti‐PSD95 and anti‐GluA1 antibodies were used to verify equal protein loading.
-
BSchematic representation of the control experiments (left panel). To rule out the possibility of stickiness of the labeled amino acids, SN were inactivated at 80°C for 5 min or pretreated with EDTA (50 mM) to disrupt polyribosomes. Next, synaptoneurosomes (untreated, heat‐inactivated, and EDTA‐treated) were NMDA‐R‐stimulated and incubated with radioactive 35S‐methionine/cysteine mix for 1 h. SDS–PAGE autoradiography shows newly synthesized proteins in SN, labeled with [35S]. Heat inactivation of SN as well as EDTA treatment inhibited 35S‐methionine/cysteine incorporation into SN.
-
C, DSynaptoneurosomes were stimulated in the presence of protein synthesis inhibitors (cycloheximide, anisomycin) (C) or VOA mixture (containing 1 μM valinomycin, 20 μM oligomycin, 8 μM antimycin) (D). Inhibition of 35S‐methionine/cysteine incorporation into de novo synthetized proteins in synaptoneurosomes was observed.
Source data are available online for this figure.
