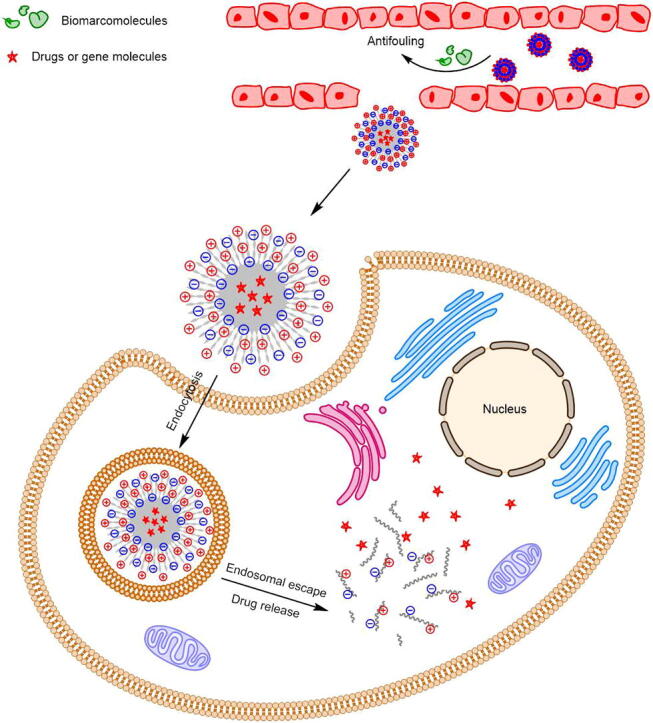
Graphical abstract
Keywords: Zwitterion, Biomaterials, Antifouling, Gene delivery, Drug delivery
Abstract
Zwitterions consist of equal molar cationic and anionic moieties and thus exhibit overall electroneutrality. Zwitterionic materials include phosphorylcholine, sulfobetaine, carboxybetaine, zwitterionic amino acids/peptides, and other mix-charged zwitterions that could form dense and stable hydration shells through the strong ion–dipole interaction among water molecules and zwitterions. As a result of their remarkable hydration capability and low interfacial energy, zwitterionic materials have become ideal choices for designing therapeutic vectors to prevent undesired biosorption especially nonspecific biomacromolecules during circulation, which was termed antifouling capability. And along with their great biocompatibility, low cytotoxicity, negligible immunogenicity, systematic stability and long circulation time, zwitterionic materials have been widely utilized for the delivery of drugs and therapeutic genes. In this review, we first summarized the possible antifouling mechanism of zwitterions briefly, and separately introduced the features and advantages of each type of zwitterionic materials. Then we highlighted their applications in stimuli-responsive “intelligent” drug delivery systems as well as tumor-targeting carriers and stressed the multifunctional role they played in therapeutic gene delivery.
1. Introduction
Over the past decades, large quantities of novel materials have been widely investigated and developed for drug and gene delivery to maintain their structural stability, alleviate cytotoxicity towards normal tissues, improve the pharmacokinetic profile and promote therapeutic efficacy [1]. However, nonspecific biomolecular and microorganism adherence is still a challenge obstructing their application in disease diagnose and treatment. Therefore, it is significant to find a method which can not only meet the requirements of nanodrug vehicles, but also resist undesired bio-adsorption effectively. The ability to avoid biosorption is termed antifouling property. Contributing to the character that can increase water solution and resist undesired adherence of biomacromolecules, poly(ethylene glycol) (PEG) has become the most commonly used biocompatible antifouling material to prevent the interactions with blood components, and prolong the in vivo circulation time [2], [3], [4]. However, it was reported that PEGylations would lose their protein repulsive capability at temperatures above 35 °C [5]. And PEG has been found susceptible to oxidation in the presence of oxygen or transition metal ions, as its non-ionic structure damage, resulting in the loss of function in most biochemically relevant solutions [6]. Aside from that, PEG was proved to be difficult to metabolize naturally, and repeated injection of PEGylated preparations would reduce the bioactivity of loaded biomolecules due to the antibodies produced by immune system [7]. Additionally, the gene entrapment efficiency of PEGylated cationic liposomes would decrease due to the decline of positive charges [8], [9], [10]. Hence, it is necessary to find alternative materials that can not only resist biosorption during blood circulation but can also keep stable in different environment, be biodegraded via metabolism and have negligible immunogenicity other than PEG.
Recent years, zwitterionic materials have attracted much attention in the field of biomaterials for their superhydrophilicity, biocompatibility, antifouling property and some other unique advantages [11]. Characterized by high dipole moment and highly charged groups, zwitterionic materials simultaneously possess an equimolar number of cationic and anionic moieties, maintaining overall electroneutrality and high hydrophilicity [12], [13]. Unlike PEG binding water molecules by hydrogen-bond interaction, zwitterions could form stronger hydration shells through ion–dipole interaction with denser and tighter adsorbed water [14], which finally leads to the ultralow of nonspecific protein adsorption, bacterial adhesion, and biofilm formation, making zwitterions preferable substitutes for PEG [15].
In addition to their marvelous antifouling property, zwitterionic materials can also enhance biocompatibility, reduce immune response, prolong the circulation time as well as promote cellular uptake of traditional chemical drugs and therapeutic genes. And zwitterionic modifications could even endow various special functions to common drug carriers, such as stimuli-responsive and tumor targeting abilities. In the following content, we will describe the features of diverse zwitterionic materials, introduce their unique advantages and summarize their applications in gene and drug delivery systems.
2. The antifouling mechanism of zwitterions
Biofouling is the spontaneous but unexpected, non-specific adsorption of biomolecules, cells or bacteria on the surface of materials [16]. In the blood, serum proteins are the main force of biosorption, which always leads to the phagocytosis and metabolism of foreign substances by reticuloendothelial systems (RES) [17]. For nanomedicines, it could greatly shorten their in vivo circulation time and results in unsatisfied therapy effect [18]. This kind of protein adhesion happens through electrostatic and hydrophobic interaction with charged segments and non-hydrophilic pockets in proteins [19]. Therefore, various approaches have been investigated to improve the antifouling properties, and the basic principle to design antifouling materials is to avoid electrostatic and hydrophobic interaction with biomolecules, which usually requires the overall neutral surface charge, remarkable hydrophilicity, hydrogen bond acceptors but no hydrogen bond donors [20].
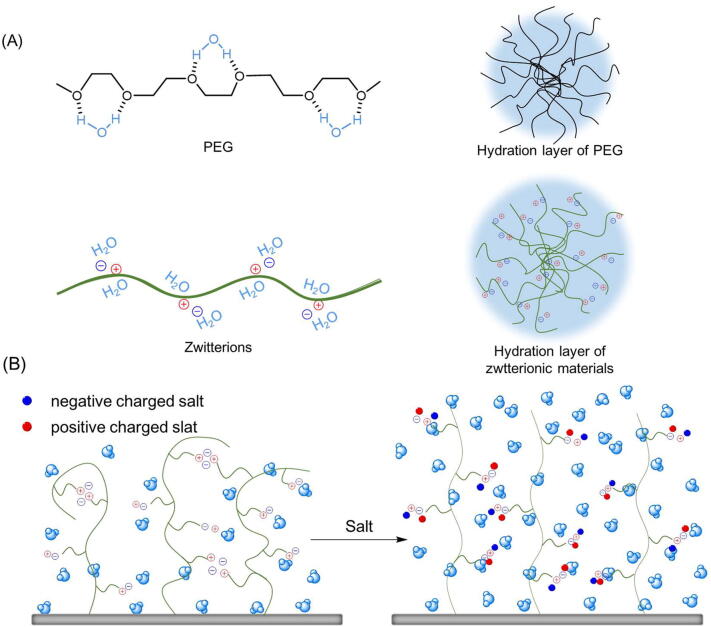
For a long time, PEGylation of nanocarriers has been considered to be the standard anti-adsorption strategy for the hydration layer formed by hydrogen bonding [21], [22]. However, this type of interaction is not strong enough that even at a high PEG grafting density, it is difficult for materials with simple PEG layers to eliminate protein adsorption completely [23], [24], [25]. Unlike PEG, zwitterions could form denser and more stable hydration shells through a totally different way. On the one hand, since zwitterions have a large amount of cationic and anionic groups, their ion–dipole interaction with water molecules brings them super hydrophilicity and high hydration capability [26]. This type of coulombic force is more powerful compared with hydrogen bonding, so that zwitterionic materials could form stronger water shells than that of PEGylations to prevent proteins form adhering [19]. On the other hand, in saline solution such as the physiological environment, the charged groups on zwitterionic polymers could attract counterions in solution to counteract the internal electrostatic force of the zwitterionic brushes, and change their conformation from shrinking to stretching relatively [27], [28], [29]. As the result of that, the hydration ability becomes more significant and brings about an enhanced antifouling property. Moreover, since the positively and negatively charged groups of zwitterions are equimolar, the overall electroneutrality further reduces the chances of nonspecific electrostatic bonding. For better understanding, the schematic diagram of hydration layer formation is showed in Fig. 1.
Fig. 1.
Schematic diagram of hydration shell formed by PEG and zwitterions separately. (A) PEG interact with H2O molecules via hydrogen bonding, while zwitterions attract H2O molecules through the powerful ion–dipole interaction, so that to form stronger hydration layers to prevent bio-adherent. (B) Incorporation of salt disrupts the previous electrostatic attraction of the charge pairs of intra-monomer, intra-chain and inter-chain, making the conformation of zwitterionic brushes from shrinking to stretching relatively.
3. Classification of zwitterionic materials
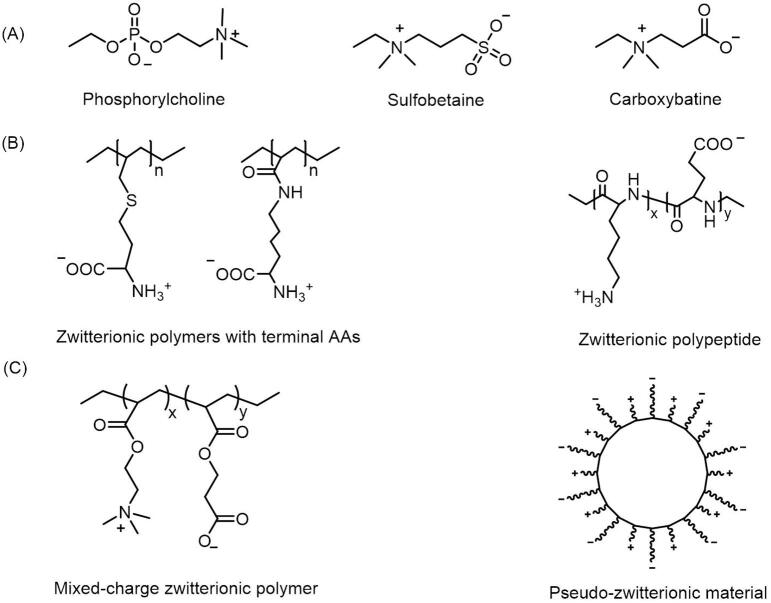
According to whether the cationic and anionic groups are on the exact same unit of zwitterions, these materials can be roughly classified as betaine-like zwitterions and mixed-charge zwitterionic materials (Fig. 2). Most of zwitterionic polymers belong to the former, they always take quaternary ammonium as cations to constitute phosphorylcholine (PC), sulfobetaine (SB) and carboxybetaine (CB) with phosphonates (PO3-), sulfonates (SO3-) and carboxylates (COO–) respectively. Except for zwitterionic polymers containing both charged segments on the same side chains, there are some mixed-charge materials containing balanced positive and negative charged moieties in different monomer units or binding to the same media (e.g. mesoporous silica nanoparticles) to maintain the overall electrical neutrality. These “spurious” zwitterionic materials own similar antifouling properties because of their semblable structures. Besides, since amino acids are a category of natural zwitterions, researchers have managed to introduce amino acids or peptides to develop zwitterionic carriers, which also exhibited great nonspecific absorption resistance and some unique properties.
Fig. 2.
Models and structures of zwitterionic materials. (A) Chemical structures of the common zwitterionic groups. (B) Zwitterionic poly(amino acids) and polypeptide. (C) Mixed-charge zwitterionic polymers that have balanced cationic and anionic groups in different monomer units, and pseudo-zwitterionic materials with equimolar negative and positive charge binding to the same medium.
3.1. Betaine-like zwitterions
3.1.1. Phosphorylcholine
PC is a zwitterionic polar group of phospholipids which play an important role in cell membranes. Therefore, PC is promising for biomimetic materials, and PC-containing nanostructures have been proved to enter living cells through fusogenic interaction with the plasma membranes [30], [31]. Ever since the famous PC-type monomer 2-methacryloyloxyethyl phosphorylcholine (MPC) was synthesized by Ishihara et al. in 1990, MPC has been utilized to build a variety of zwitterionic polymers for its great biocompatibility and strong hydration caused by electrostatic interactions [32]. About 14 years after that, Ishihara and his team reported the first PC-modified drug delivery system (DDS), poly(2-MPC-co-n-butyl methacrylate) (PMB), which was designed to carry the poor hydrophilic paclitaxel (PTX) [33]. The zwitterionic MPC served as hydrophilic segments, which could make the copolymers self-assemble into micelles in aqueous solution together with hydrophobic parts. Thus, the water solubility of PTX was enhanced, and its stability in the blood was also improved taking advantages of the antifouling property of zwitterionic MPC [12], [34]. However, it is not easy to synthesize and handle PC-based polymers in general since their monomers, such as MPC, are moisture sensitive, and studies found out that their phosphoester groups had a tendency to be hydrolyzed, which would shorten their in vivo circulation time and badly obstruct application for drug or gene delivery [35], [36].
3.1.2. Sulfobetaine
Betaine is a type of natural zwitterionic molecule widely existing in many plants. As a derivate of it, SB has the characteristics of common zwitterions and exhibits extremely remarkable hydrophilia. By means of differential scanning calorimetry, researchers found that there are 8 mol H2O bound to 1 mol SB group, which is far more than a single unit of PEG [37], [38]. For that reason, SB has attracted growing attention with its good biocompatibility and resistance to nonspecific protein adsorption. Recent years, several studies have attempted to introduce SB groups to delivery systems, in which the modified carriers exhibited effective drug loading, high cellular uptake rate, tiny cytotoxicity and faint immune response. For instance, researchers developed a starch-based polymer SB-ST-OC (SSO) with hydrophobic octane groups (OC) and zwitterionic SB, and the results proved that SSO micelles had less macrophage activation potential compared with ST-OC and naked DOX, through detecting the secretion of TNF-α and IL-6 on the macrophage phagocytosis of micelles [39]. And another important reason why SB-coupled materials, especially sulfobetaine methacrylate (SBMA), become so attractive should be the lower synthetic cost compared with PC-modified ones.
Since the strong charge-charge or dipole–dipole interactions among the zwitterionic sulfobetaine moieties, SB-based polymers usually exhibit a sharp phase transition at the upper critical solution temperature (UCST) in aqueous solution [40]. That means their solubility would be influenced by the polarities and ionic concentration of solvent [41], [42], [43], [44], and by adjusting the solvent conditions, the UCST could be modulated to a desired temperature to build thermo-responsive polymers [45].
3.1.3. Carboxybetaine
CB could be one of the most popular zwitterions in the last few years. Similar to SB, CB is also a derivate of the betaine that is ultralow-fouling characterized. Although SB and CB are alike in structures and are both good at resisting undesired adherence, the difference between their anionic groups (COO− for CB and SO3− for SB) makes these two zwitterions have diverse properties in hydration, ionic interaction, and self-association [12]. As mentioned above, the solubility of PSB depends on the specific conditions of solvents, while polycarboxybetaine (PCB) has been proved to have remarkable hydration properties in a wide range of media [12]. And scientists found that the cationic and anionic groups of CB segments formed few inter-/intra-associations, so that CB-grafted polymers hardly exist thermal or salt responses caused by the self-associations among zwitterionic moieties, whereas SB polymers do [41]. More importantly, the carboxylate anion groups of PCB are able to be functionalized for the immobilization of amine-containing biomolecules including some antibodies, targeting ligands and fluorescent dyes, allowing PCB and its derivatives multifunctional materials [46].
3.2. Mixed-charge zwitterionic materials
3.2.1. Zwitterionic amino acids and peptides
Amino acids (AAs) are natural zwitterions that have both positive charged amino groups and negative charged carboxyl groups on the α-carbon atoms. AAs possess the common advantages zwitterionic materials have, so it is valid to conjugate them as terminal groups with conventional polymers that are weak in undesirable biosorption resistance or stability [47]. Some basic AAs including lysine (Lys), histidine (His) and arginine (Arg) have one more amine group, while some AAs such as aspartic acid (Asp) and glutamic acid (Glu) have one more carboxyl group. What makes it special is that these types of AAs have redundant functional groups, so they could form peptide bonds with α-carboxyl/amino groups to get mixed-charge zwitterionic peptides, or perform the dehydration condensation reaction with their redundant carboxyl/amino groups to get betaine-like zwitterionic side chains with terminal AAs (Fig. 2B). Since the zwitterionic polypeptides (ZIPPs) are another kind of biomimetic materials that are similar to the surficial proteins on cellular membranes, they generally exhibit outstanding biodegradability, biocompatibility and in vivo stability [48]. Even more, the amino terminals and carboxyl ends make it possible to introduce functional biomolecules just like CB-based materials, so zwitterionic AAs and polypeptides are great choices for drug/gene delivery requiring abundant bio-functions such as tumor targeting.
3.2.2. Other mixed-charge zwitterionic materials
In addition to the mixed-charge zwitterionic peptides, zwitterionic polymers could also be synthesized with different components bearing equivalent opposite charges to maintain the overall electroneutrality (Fig. 2C) [49]. For example, taking zwitterionic amino acid decorated amphiphilic DDSs as models, poly(aminoethyl methacrylate)-co-poly(methacrylic acid)-co-poly(n-butyl methacrylate) (CPMA) was developed for the delivery of doxorubicin (DOX) [50]. Although the cationic amino groups and anionic carboxyl groups of this copolymer were distributed in the different side chains, they still owned excellent antifouling property, biocompatibility as well as biostability. What’s more, trough linking electric-contrary low-weight molecules to a electroneutral media, researchers have designed a series of mixed-charge pseudo-zwitterionic materials whose characteristics were proved similar to the ordinary zwitterionic ones, some of them were even given novel functions [51], [52].
4. Applications of zwitterionic materials in drug delivery systems
As mentioned at the beginning, more and more zwitterionic materials are applied to carry therapeutic agents for their ultra-antifouling property and wonderful biocompatibility. But that alone is far from enough to meet clinical demands, since competent carriers should also have great loading volumes, promote cellular uptake, and control the drug release as expected. Therefore, sorts of “intelligent” carriers have been engineered to undergo corresponding changes in material properties when exposed to different biological stimuli [53], so that they are able to protect fragile cargoes until they reach the targets and unload them effectively at active sites. The zwitterionic drug delivery systems involved in this review are shown in Table 1.
Table 1.
Summary of stimuli-responsive zwitterionic DDSs.
| Category | Polyampholytes | Working morphology | Loaded drugs | Special properties | Ref. |
|---|---|---|---|---|---|
| PC | PMB | micelles | PTX | – | [33] |
| PMPC-PDPA | micelles/vesicles | PTX/DOX | pH-responsive | [61], [63] | |
| NAcHis-PCCs | NPs | quercetin | pH-responsive | [73] | |
| PMPC-PLA | vesicles | DOX/DOX·HCl | pH-responsive | [85] | |
| PMPC-Blink-CPLA | micelles | PTX | pH-responsive | [86] | |
| PLL-PMPC(DOX) | micelles | DOX | pH-responsive | [87] | |
| PMPC-PCL | micelles | DOX | – | [104] | |
| PMPC-6sPCL | micelles | PTX | – | [105] | |
| PMPC-ss-PCL | micelles | DOX | redox-responsive | [106] | |
| PMPC-TMZ | micelles | TMZ | redox-responsive | [115] | |
| FA-MPC-DPA | micelles | tamoxifen, PTX | pH-responsive tumor-targeting | [136] | |
| FAMEG-MPC-PCL | micelles | DOX | tumor-targeting | [140] | |
| SB | SSO | micelles | DOX | – | [39] |
| PSBMA-PDEA-PCL | micelles | CUR | pH-responsive | [68] | |
| PSBMA | nanogels | DOX | redox-responsive | [110] | |
| PCL-ss-PDEASB | micelles | DOX | pH- and redox-responsive | [119] | |
| PSBMA-PDPA | micelles | CUR | pH- and thermo-responsive | [127] | |
| CB | PCBMA-ss-PCL-ss-PCBMA | micelles | DOX | redox-responsive | [108] |
| PCBMA-PMAEL | micelles | DOX | redox-responsive | [111] | |
| PCBMA(DC-C) | NPs | DOX·HCl | pH- and redox-responsive | [120] | |
| P(CBMA-DMAEMA)@ZnO | NPs | DOX | pH-responsive | [122] | |
| cRGD-PCSSL | NPs | DOX | redox-responsive tumor-targeting | [145] | |
| cRGD-PCSSD | NPs | DOX | pH-, redox-responsive tumor-targeting | [152] | |
| Poly(AAs) and polypeptides | HHG2C18-L | liposomes | coumari6/temsirolimus | pH-responsive | [75], [77] |
| P(Lys-alt-N, N′-bis(acryloyl) diaminohexane) | micelles | DOX | pH-responsive | [76] | |
| M-HHG2C18-L | NPs | Erlotinib/DOX | pH-responsive | [79] | |
| PALHy-hyd-DOX | micelles | DOX | pH-responsive | [92] | |
| P(lysine methacrylate) | micelles | CPT | redox-responsive | [116] | |
| cRGD-PMIX | vesicles | DOX | pH-responsive tumor-targeting | [146] | |
| Mix-charged zwitterions | CPMA | micelles | DOX | pH-responsive | [50] |
| MSN-HA-SiO2-TSA/DMA | NPs | DOX | pH-, redox-and HAase-responsive | [51] | |
| Z-MSN | NPs | DOX | pH-responsive | [52] | |
| MCM-41 | NPs | LEVO | pH-responsive | [81] | |
| dHAD | micelles | DOX | pH-responsive tumor-targeting | [155] |
4.1. pH-responsive zwitterionic DDSs
The inherent differences of pH value among different tissues, organs and cellular compartments make pH an attractive factor to control drug delivery according to diverse internal environment [54], [55], [56]. It’s well known that the tumor microenvironments (pH 6.4–6.8) are always more acid than that in bloodstream and normal tissues (pH 7.4) [57], taking use of it could promote the accumulation of drugs around tumor cells to reduce side effects. And since the pH decreases from pH 7.4 to pH 5.5–6.0 in endosomes and even below pH 5.0 in lysosomes, it could act as a trigger for drug release by lowering the pH during endocytosis [58]. Therefore, scientists have developed a series of pH-responsive zwitterionic carriers to protect drugs from non-specific adsorption in bloodstream, prevent cargo premature leakage during circulation and help drugs escape from endosomes/lysosomes rapidly with their morphological or chemical changes at target sites [59].
To design pH-responsive carriers, one of the most common strategies is using charge shifting polymers that would change in conformation or solubility according to the pH value of the surrounding environments. Armes and his partners synthesized a series of zwitterionic amphipathic block copolymers based on poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) and the pH-sensitive poly(2-(diisopropylamino)ethyl methacrylate) (PDPA) through atom transfer radical polymerization (ATRP) method [60], [61], [62]. The PDPA blocks would be protonated and hence hydrophilic under dilute acidic environment but become deprotonated when adjusting the solution to physiological pH value. This would lead to the self-assembly of PMPC-PDPA micelles in bloodstream, and loaded the water insoluble cargoes by the hydrophobic PDPA core [61]. When the micelles were endocytosed into cells, PDPA blocks would be protonated and cannot maintain the micelle structure, which was followed by quick drug release in the cytoplasm. By adjusting the polymerization degree of PMPC and PDPA, the drug release kinetics could be regulated, as well as their working morphology (micelles, vesicles, etc.) [61], [63]. In fact, Armes and his co-workers also attempted to use PMPC-PDPA as novel non-viral vectors for gene delivery [64]. Through the electrostatic interactions between the cationic groups and negative charged nucleic acids, genes are condensed by these zwitterionic polymers. And the MPC moieties could provide a “stealth” shell to protect the loaded pDNA from promiscuous absorption, and the DPA segments endowed the vectors pH-responsive capability [64]. It has been verified that about 90% of the loaded DNA could be released from the PMPC-PDPA, with pH switching from physiological level (pH 7.4) to endocytic (pH 6.0) level [65], [66]. The PMPC-PDPA copolymer was also used to transfect siRNA to knockdown MDM2 gene in p53 mutant cells for cancer inhibition, and the animal experiment showed a significant effect in cell cycle arrest, apoptosis and growth inhibition [67].
Similarly, by replacing the DPA block with other tertiary amine containing monomers, numerous pH-sensitive zwitterionic polymers have been developed. For example, an amphiphilic triblock copolymer, poly(ε-caprolactone)-poly(diethylaminoethyl methacrylate)-poly(sulfobetaine methacrylate) (PSBMA-PDEA-PCL) has been synthesized [68]. The PDEA block worked as a pH-responsive switch, owing to its amphoteric nature by protonation-deprotonation brought from unsaturated nitrogen of tertiary amine [68], [69]. However, PDEA is so sensitive to environmental pH that might influent stability of the core, and the cytotoxicity caused by its charge property also disturbed its application [70]. To solve this problem, PSBMA was combined with PDEA to maintain stable micelles and limit its toxicity, in the meanwhile to serve as a hydrophilic protective layer to resistant nonspecific biosorption.
In addition, the unsaturated nitrogen of the imidazole group in histidine would be protonated to become hydrophilic, and the size of histidine containing self-assembled nanoparticles (NPs) will significantly increase due to the breakdown of the hydrophobic/hydrophilic balance [71]. Wu et al. respectively linked pH-responsive N-acetyl-L-histidine (NAcHis) and membrane-like PC groups to chitosan, a typical polysaccharide that can be disintegrated in vivo [72], to prepare NAcHis-PCCs conjugate [73]. The PC shell provided excellent antifouling property and biocompatibility to avoid adverse biological response. And since the pKa of the imidazole group in histidine is around 6.5, NAcHis would be protonated to be hydrophilic and destabilized the nanocarriers which eventually led to cargo release when the milieu pH < 6.5. By using Quercetin (QUE) as a model drug, the scientists found an acid-triggered behavior of NAcHis-PCCs that the release efficiency of QUE under acidic conditions (pH 5.5) was enhanced compared to physiological conditions (pH 7.4), which consisted well with the pH-responsive size changing behavior measured by dynamic light scattering (DLS).
Since some amino acids have extra amino/carboxy groups that can act as charge-reversing switch, researchers attempted to add AAs into zwitterionic polymers for pH-sensitive drug delivery [74], [75]. For example, a pH-sensitive charge-reversal zwitterionic copolymer containing basic lysine, poly(Lys-alt-N,N’-bis(acryloyl) diaminohexane) was synthesized through the Michael addition polymerization between N,N’-bis(acryloyl) diaminohexane and lysine [76]. This amino acid-based copolymer could self-assemble into nanomicelles and show a slightly negative charge in blood but exhibit a positive charge in acid environments. Because of the high protonation of tertiary amino groups in the backbones and zwitterions of side chains in nanomicelles under the acidic condition, the hydrodynamic radius of the nanomicelles would increase thus to facilitate the encapsulated DOX diffusion at pH 5.0.
Similarly, Takeoka and co-workers developed two amino acid-based lipids with different head groups, in which 1,5-dihexadecyl N-glutamyl-L-glutamate (L1) and 1,5-dihexadecyl N,N-diglutamyl-lysyl-L-glutamate (L2) were served as pH-responsive components to form charge-converse DOX-encapsulating liposomes [74]. The L2 moiety had two glutamic residues while the L1 had only one, both of the L1- and L2-liposomes exhibited good pH-responsibility as well as improved membrane fusogenic potential, but the DOX-loading efficiency of L1-liposomes was lower than the other ones for the distinction of head groups. Hence L2-liposomes was chosen to be intravenously injected to model rats, displaying comparable blood persistence and biodistribution to conventional phosphatidylcholine-based liposomes, but a higher toxicity to Hela cells.
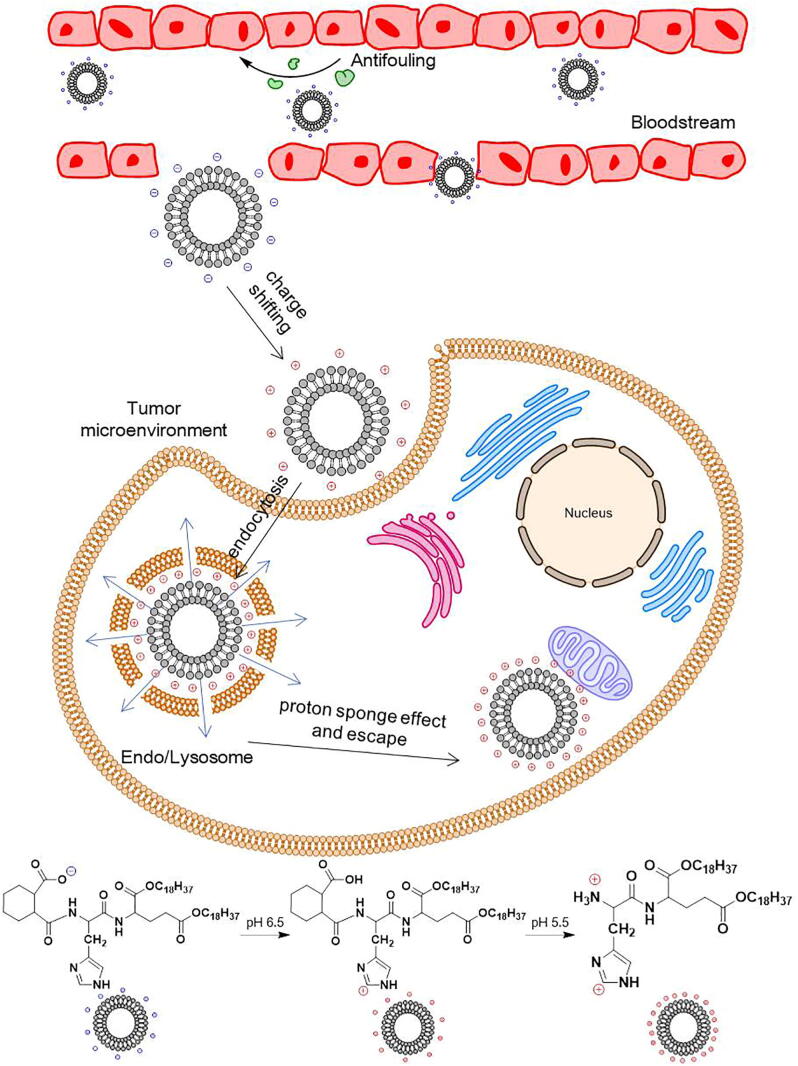
Also, Zhang et al. synthesized a pH-responsive liposome based on zwitterionic oligopeptides that were able to convert the electrical property according to different pH in tumor matrix and intracellular compartments [75], [77]. Besides common phosphatidylcholine and cholesterol in normal liposomes, this oligopeptide-based zwitterionic lipid (HHG2C18-L) employed glutamine acids (G), histidine (H) and pH-cleavable hexahydrobenzoic amide groups as its hydrophilic block, while stearyl alkane chains (-2C18H37) as the hydrophobic block. After the HHG2C18-L liposome arriving at tumor sites (pH 6.5) and convert surface charges from negative to positive ones, the cellular uptake could be enhanced via electrostatic interaction. And the imidazole groups of histidine promoted proton influx to endosomes/lysosomes, resulting in their bursting. As pH got lower, hexahydrobenzoic amide would be hydrolyzed, which brought about even stronger positive surface charge and escaped from endo/lysosomes to release the renal cancer-against drug temsirolimus eventually (Fig. 3). Furthermore, with the constant further studies on combination chemotherapy, “drug cocktail” has become an optimal therapeutic strategy for cancer treatment. Studies demonstrated that pretreatment of lung carcinoma cells using erlotinib could significantly synergize with DOX [78], but this combination needed a specific time lag between the drugs to maximize the effect, which asked an adaptive DDS to match the pharmacokinetic parameters for each drug and control their release sequence preciously. To fit for the “drug cocktail” method, Zhang’s group constructed a charge reversal erlotinib/DOX co-delivery nanoparticle (M-HHG2C18-L) by using their pH-sensitive lipid bilayer HHG2C18-L as a coat to cover amino-functionalized mesoporous silica nanoparticles (MSNs) [79]. They entrapped DOX in MSN-NH2 followed by coverage with erlotinib-carried lipid bilayer, so that the exterior EGFR inhibitor would release faster than DOX. This pH-sensitive charge conversion co-delivery system not only possessed all the properties of HHG2C18-L, but also achieved staggered sequential drug release without any damage to other major organs, showing great potential for cancer therapy.
Fig. 3.
The multistage pH-responsive process of the smart liposomes (HHG2C18-L). HHG2C18-L kept negative charged in bloodstream. When the liposomes arrived at tumor site by EPR effect, their surface charge would convert to positive through the protonation of the imidazole group in histidine thus to promote endocytosis. As the proton swarmed into endo/lysosomes, the proton sponge effect led to endo/lysosomal bursting and facilitated liposome escape. The following hydrolysis of hexahydrobenzoic amide brought about an extremely strong positive surface charge of by abandoning the carboxy groups in liposomes, which obstructed the charge reverse again when escaped to cytoplasm. Eventually, the positive charged HHG2C18-L might accumulate at the mitochondria via electrostatic interaction attributing to the mitochondrial transmembrane potential.
In addition, a great number of mixed-charged zwitterionic materials are designed based on MSNs, which have good biocompatibility, tunable pore sizes, modifiable surface and remarkable drug loading capacity [80]. To improve drug loading efficiency and cellular uptake, a zwitterionic MSN (Z-MSN) was prepared by introducing a composition of carboxylic groups and quaternary amino groups as gatekeepers responding to tumor microenvironment [52]. The surface charge of Z-MSN would switch from negative to positive with cleavage of the acid-sensitive amide linkages, enhancing effective uptake of DOX into tumor issues, leading to much higher antitumor effect compared with free DOX. And a Spanish team designed a mixed-charge zwitterionic MSN (MCM-41) whose external surface was decorated with short chains of aminopropyl silane triol and trihydroxy-silyl-propyl-methyl phosphonate mixed-charge brushes [81]. Levofloxacin (LEVO) was loaded into the pores of MCM-41, which displayed a remarkable antimicrobial activity against pathogenic E. coli and S. aureus in a low-fouling and reduced macrophage-uptake method.
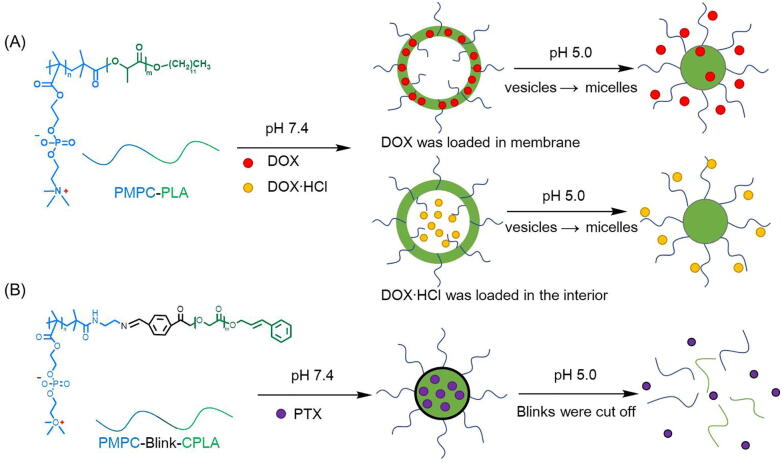
Another strategy to design pH-responsive zwitterionic carriers is introducing acid-labile linkages (such as hydrazine, acetal and imine) that could be cleaved at acid environments but stable at neutral pH [55], [82], [83]. Liu et al. at first used a common aliphatic biodegradable polyester poly(D,L-lactide) (PLA) as hydrophobic segments to synthesize diblock copolymer PMPC-PLA by ATRP of MPC monomer with PLA-Br as the initiator [84]. PMPC-PLA vesicles were prepared via a dialysis method and DOX/DOX·HCl were loaded into the vesicle membrane and interior respectively. The results showed significantly faster drug release at pH 5.0 compared to pH 7.4, which might attribute to the hydrolysis of PMPC-PLA, resulting in morphology transformation from vesicle to micelle thus to extrude the drugs (Fig. 4) [85]. Later, they introduced pH-sensitive benzoyl imine linkage (Blink) in PMPC-PLA as a bridge to get a new diblock copolymeric micelle for PTX delivery. And the PLA block was ended by cinnamyl alcohol (CPLA) whose benzene rings could interact with PTX via π-π stacking to improve the loading content [86]. The in vitro study showed over 90% of PTX were released from the PMPC-Blink-CPLA micelles in 48 h at pH 5.5, which was ascribed to the cleavage of their acid-labile switch Blink.
Fig. 4.
The process of drug loading and release from PMPC-PLA and PMPC-Blink-PLA. (A) Hydrophobic DOX and hydrophilic DOX·HCl was loaded in the membrane and interior of the PMPC-PLA vesicles respectively. When pH decreased from 7.4 to 5.0, the hydrolysis of PLA segments would lead to morphology transformation from vesicles to micelle, and finally extruded the encapsulated drugs. (B) The PMPC-Blink-CPLA self-assembled into micelles and encapsulated PTX by CPLA core via hydrophobic interactions as well as π-π stacking. The pH-sensitive benzoyl imine linkages would be cut off in acid environment and the disruption of micelles finally resulted in drug release.
Except for being used as block linkages, diverse polymer-drug conjugates (also called prodrugs) have been developed through connecting drugs and carriers with some suitable chemical bonds that can not only maintain the micelles structure, but also hold drugs firmly with neglectable drug leakage during blood circulation than traditional micelles [83]. In order to enable the prodrug response to environmental pH value, Wang et al. developed zwitterionic prodrug PMPC-PLL(DOX) using poly(L-lysine) as the functional segment to connect DOX via imine bonds. The PMPC-PLL(DOX) prodrug could self-assemble into core–shell structural stable micelles under physiological environment, but when transferred to the acidic environment (pH 5.5), their imine bonds would be cut off to reverse surface charge so that contributed to the endosomal escape and accelerated drug release [87].
Similarly, quantities of prodrugs have been synthesized with the help of hydrazone bonds which are easy to be cut off in acid environments too [88], [89], [90], [91]. Wu et al. conjugated zwitterionic L-Lys and hydrazine grafted poly(aspartamide) (PALHy) by successive aminolysis reactions of poly(succinimide) with L-lysine and hydrazine hydrate following hydrolysis [92]. The DOX molecules were conjugated to the copolymer chemically via hydrazone bonds to form PALHy-hyd-DOX conjugates. Performing as a hydrophobic moiety, DOX could trigger self-assemble of the nanoparticle in aqueous solution, which resulted in hydrophobic interactions as well as π-π stacking to encapsulate more dissociative DOX molecules subsequently.
Taking advantages of the acid-labile characteristics of acetal segments, Wang et al. fabricated a pH-sensitive SB-modified dendrimer (Gn’-SB) for antitumor drug delivery [93]. Dendrimers are regarded as a novel kind of promising micelle in the field of DDSs owning to the unique merits of uniform architectures, controllable sizes, abundant terminal groups, huge internal cavities, surface functionality and low intrinsic viscosity in solutions [94]. They selected polyacetal as the acid-labile segment for its ability to be hydrolyzed in an acidic environment with non-acidic metabolites, which makes it biodegradable [95], [96]. And zwitterionic SB was conjugated to the tails of polyacetal to promote endocytosis by tumor cells for its high affinity to negatively charged cytomembranes in faintly acid conditions [60].
4.2. Redox-sensitive drug carriers
In addition to taking advantage of the pH difference, the usual stimulating factors include redox potential, temperature, light, specific enzymes and magnetic field [97], [98], [99], [100]. Due to the overexpression of glutathione (GSH) in the intracellular components of tumor cells (10 mM) which could be much higher than that of the extracellular fluid and blood (10 μM), it is possible to achieve faster tumor intracellular drug release triggered by GSH [101], [102]. And since disulfides could be easily reduced to sulfhydryl group under the reduction circumstance, disulfide linkages were widely utilized to build redox-sensitive therapeutic delivery systems [103].
At the outset, amphiphilic diblock polymers based on zwitterionic PMPC and biodegradable poly (ε-caprolactone) (PMPC-PCL) with different polymerization degree were developed as substitutes for PEG-PCL. Both of them could load hydrophobic drugs efficiently, but PMPC-PCL micelles exhibited longer circulation time and higher accumulation rate by tumor cells attributing to the strong hydration and bioinspired effect of PMPC [104], [105]. However, the peripheral dense shells prevent the enzymolysis of PCL core, which is not conducive to drug release. Thereafter, scientists introduced disulfide bond as a bridge between PMPC and PCL moieties so that the new block polymer (PMPC-ss-PCL) could be broken up and unload cargoes rapidly in the presence of high GSH concentration in tumor cells. Cai et al. found an obvious size increase of the PMPC-ss-PCL micelles in presence of 10 mM dithiothreitol (DTT, another reducing agent to mimic intracellular tumor microenvironment) but no significant size change for 16 h in absence of DTT [106]. This phenomenon might happen because the disulfide bonds were cut off by DTT, so the PMPC shell pulled off from the micellar surface leading to micelle aggregation [107]. In this research, DOX-loaded PMPC-ss-PCL micelles showed faster drug release under reducing conditions in vitro, and much better anti-tumor effect than no-sensitive ones and positive DOX·HCl. Analogously, a reduction-sensitive triblock zwitterionic polymer PCBMA-ss-PCL-ss-PCBMA was fabricated by connecting hydrophilic PCBMA and lipophilic PCL groups with disulfide bonds [108]. Compared to a diblock polymer, this triblock one contains more disulfides and zwitterionic moieties in one unit, which may bring stronger anti-biosorption and redox-responsive properties.
Except for being used as linkages to build block polymers, disulfide bonds could also act as cross-linkers to form reduction-responsive cross-linking structures which have emerged as a feasible strategy to minimize premature drug release and facilitate unloading within tumor cells [109]. For instance, a biodegradable zwitterionic PSBMA nanogel was synthesized by one-step reflux precipitation copolymerization of SBMA and N,N’-bis-(acryloyl) cystamine (BAC) [110]. DOX was held firmly by PSBMA nanogel in physiological conditions with only 7% leakage for 24 h, but 85% release in 8 h under a high GSH environment by the cleavage of the disulfide bonds in BAC. After intravenous injection in human hypopharyngeal carcinoma-bearing mouse models, the PSBMA nanogel showed excellent properties including high tumor accumulation, excellent biodegradation, long circulation, and sufficient drug release. And to enhance the stability of zwitterionic amphiphilic copolymers, Chen’s team synthesized a random copolymer P(CMBA-co-MAEL) by using zwitterionic carboxybetaine methacrylate as hydrophilic part and disulfide containing 2-(methacryloyloxy) ethyl lipoate as hydrophobic segment [111]. They first used Tris(2-carboxyethyl) phosphine to reduce the disulfides from MAEL, then stirred the mixture to form shell-cross-linked micelles via O2-mediated oxidization. DOX was loaded into the carriers before the micelles were cross-linked, and it proved that the pCBMA moieties provided excellent hemocompatibility and remarkable resistance to biosorption, with selective release of DOX in reducing environments owing to the cross-linked shell.
Besides, various polymeric prodrugs linked by disulfide bonds have already been tested for chemotherapeutics [112], [113], and scientists also managed to connect therapeutic drugs to zwitterionic polymers by disulfide linkers directly to gift zwitterionic prodrugs the reduction-responsive properties. Temozolomide (TMZ) is a first-line drug for glioblastoma, but it is hydrolytic instability and is impeded by enzyme-mediated chemoresistance after crossing the blood brain barrier [114]. To improve its stability in aqueous solution, Emrick et al. synthesized a battery of PMPC-TMZ conjugates that showed enhanced drug stability as well as much longer half-life times [115]. Afterward, they expanded this delivery platform by introducing disulfide linkers to trigger TMZ release efficiently under high GSH intracellular tumor condition. And another broad-spectrum antitumor drug camptothecin was conjugated to the lysine residue side chains of zwitterionic poly(lysine methacrylate) via GSH-sensitive disulfide bonds to form a reduction sensitive polymer-drug conjugate [116]. In the meanwhile, the authors linked anisamide, a sigma-1 receptor to this polymer as a guide to target cancerous endothelial cells, but the ligands utility in tumor targeting is now controversial [117].
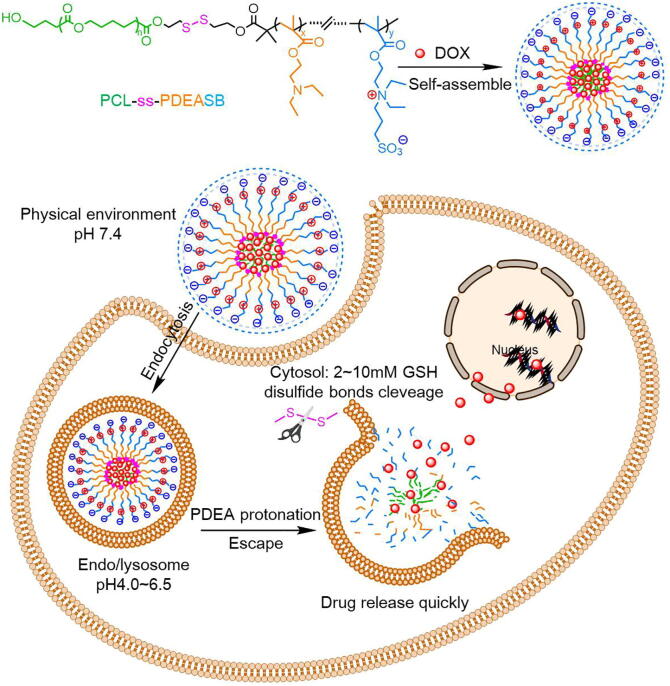
In fact, scientists soon came up with the great idea of preparing “smart” amphoteric delivery systems that can respond to more than just one stimulus to help therapeutics work more effectively [118]. The first pH and redox dual-sensitive zwitterionic DDS was synthesized by linking pH-responsive PDEA and PCL via redox-sensitive disulfide bonds, and the SB segment was added to gain the zwitterionic copolymer PCL-ss-PDEASB [119]. This amphiphilic copolymer could self-assemble into micelles and have long circulation in blood as well as effective internalization by cells owing to the SB shell. After endocytosis, the micelles could not only release the encapsulated DOX which was triggered by the protonation of PDEA groups in acid condition, but also unload the drugs quickly with the reduction of disulfide bonds in respond to intracellular high GSH concentration. Hence, the drug-loaded PCL-ss-PDEASB micelles were able to release lipophilic antitumor drugs fast under acidic and reductive conditions simultaneously to kill tumor cells efficiently, and the modification with SB made it possible even to eliminate the cytotoxicity of drug carriers with PEDA (Fig. 5).
Fig. 5.
The forming process and intracellular drug release of the PCL-ss-PDEASB/DOX system. With the shield of zwitterionic SB shell, these micelles could avoid undesired absorption, and internalized by tumor cells effectively. After endocytosis, the loaded DOX would be release rapidly as the result of PDEA protonation in response to low pH and disulfide bonds cleavage triggered by cytosol high GSH concentration.
Through a different approach, another typical pH- and redox-sensitive DDS was designed to cover peptide dendrimer-modified carbon dots by PCBMA through the interaction between the terminal guanidine on arginine dendrimers and carboxyl groups on PCBMA [120]. In this work, PCBMA not only provided “stealth” delivery in blood, but also achieved electric reverse after enriching at tumor sites. The zwitterionic coat would thus be taken-off from the PCBMA(DC-C) to expose the abundant peripheral guanidine groups with positive charges, which were beneficial to enhance endocytosis across the negative charged cell membranes. Eventually, the highly reductive and acid condition in tumor cells would cut-off the disulfide bonds between peptide dendrimer and carbon dots, desorbing DOX·HCl to accelerate the nucleus-specific drug release for cancer therapy. And PCBMA has also been utilized to decorate quantum dots to improve their properties for drug delivery. Just like ZnO quantum dots which can be decomposed into zinc ions completely in the aqueous solution with a pH of 5.0 thus become toxic to tumor cells [121], [122]. But their water insolubility and ease of agglomeration require antifouling modifications, so PCBMA-poly(2-(dimethylamino) ethyl methacrylate (PDMAEMA)) PCBMA-PDMAEMA was employed as a pH-sensitive coat to compensate for their shortcomings [122].
4.3. Thermo-responsive zwitterionic vehicles
Thermo-responsiveness is one of the most widely used stimulus that can lead to reversible phase transition with just a slight change of temperature, so that thermo-sensitive DDSs have attracted much attention to control drug release [123]. As mentioned in the classification chapter, SB-based polymers such as PSBMA exhibit a sharp phase transition below their UCST in aqueous solution owning to the strong electrostatic interactions among their SB moieties [124]. That means the combination of zwitterionic SB and other suitable non-ionic segments might achieve thermo-responsive carriers which could transit from soluble to insoluble through adjusting the environmental temperature.
Through free radical polymerization, Chen and co-workers prepared dually responsive copolymers consisting of thermo-responsive SBMA and pH-sensitive DPA copolymer for drug oral delivery P(SBMA-co-DPA) [125]. In this work, SBMA was selected owing to its strong electrostatic attraction between their ammonium cation and sulfo-anion, which made the zwitterionic copolymer self-aggregate into NPs in aqueous media within their UCSTs [124], and thus to load the drug curcumin (CUR) [126]. When the temperature increased above the UCST, collapsed associations of polymer chains would transit to the unimer state to facilitate drug release. Interestingly, the authors found the UCSTs of P(SBMA-co-DPA) varied with different solution pH and exhibited a pH-dependently thermal-responsive behavior, that might be caused by the protonation/deprotonation of DPA segments. When the environmental pH value was below the pKa of DPA moieties, the protonated amino groups brought positive charges to the copolymer which might introduce strong electrostatic repulsion to diminish the attractive interaction among SB moieties. That means the more content of DPA segments, the lower UCSTs of P(SBMA-co-DPA) would be achieved at the same pH value. So they tried different molar ratios of SBMA and DPA and results showed P(SBMA90-co-DPA10) could be well-dispersed at extremely acidic condition like stomach (pH 1.2–3.0), but at neutral pH such as intestine (pH 6.5–8.0) the NPs would be solvated as unimer and unloaded CUR since their UCST became lower than body temperature [127]. Thus, these pH-sensitive and thermo-responsive zwitterionic copolymers PSBMA-PDPA are promising to be applied as oral delivery drug carrier.
4.4. Tumor targeting zwitterionic carriers
Achieving high uptake rate by tumor cells is still a challenge demanding prompt solution for the development of antitumor drugs’ delivery which could improve the therapeutic efficiency and limit side effects as possible. To enhance the specific accumulation of drugs in tumor sites, various tumor target-specific moieties are conjugated to the surface of carriers, such as folic acids (FAs) [128], [129], mucopolysaccharide [130], peptides [131], [132], [133], and antibodies [134]. Unexceptionally, these target-specific ligands have also been widely introduced to zwitterionic vehicles so that they can be recognized by the corresponding receptors that are overexpressed on the surface of specific tumor cells.
Since FA shows extremely high affinity for the folate receptor (FR) that is known to be overexpressed on the membrane of malignant cells include ovary, cervical and nasopharyngeal carcinomas [135], FA-decorated carriers could promote cellular uptake for FR-positive cancer cells. Therefore, Armes’ team conjugated FA to the primary amine terminus of the PMPC-PDPA to endow this pH-sensitive diblock copolymer tumor targeting property [136]. They first prepared PMPC-PDPA through ATRP using an Fmoc-protected initiator and treated the purified Fmoc-MPC-DPA with 5% DBU in methanol to get the primary amine-functionalized H2N-MPC-DPA [137]. FA was then conjugated to the deblock copolymer to obtain FA-MPC-DPA, and a known amount of tamoxifen and PTX was mixed with FA-MPC30-DPA50 and FA-MPC30-DPA80 respectively, to fabricate drug-loaded micelles. Cell viability studies demonstrated that both micelles were more toxic toward tumor cells compared to non-targeting ones. And due to protonation of the DPA blocks, the micelles dissociated at pH 5.5 thereby triggering the loaded drug release rapidly.
But the method used in FA-MPC-DPA that introducing FA to the copolymer chain as a terminal group brings difficulties in controlling the content of targeting groups, since there could just be a single FA group in one copolymer [138], [139]. To solve this problem, FA-modified poly(ethylene glycol) methacrylate macromonomer (FAMEG) was first synthesized by a two-step synthetic strategy [140]. The copolymer FAMEG-MPC-PCL was then fabricated through free radical polymerization of PCL macromonomer, MPC, and FAMEG [141]. Thus, the content of FA groups in this copolymer could be expediently adjusted via changing the molar ratio of monomers.
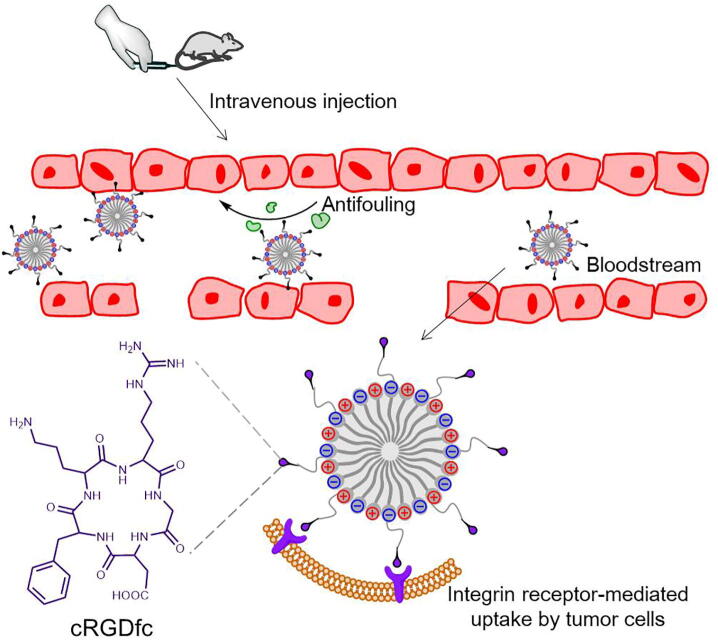
The arginyl-glycyl-aspartic acid (RGD) is an oligopeptide that expresses on many extracellular matrix proteins and plays a significant role in cell direction and cell linkage [142]. RGD could selectively recognize and bind to the αv class of integrins, which are overexpressed during tumor progression and have been regarded as tumor markers [143]. For this reason, different forms of RGD include liner and cyclic structures were widely investigated for tumor targeting [144], but the cyclic ones exhibited better activity attributing to a conformation-less assembly that was able to withstand proteolysis and had higher affinities to interact with integrin receptors [140], so that lots of cyclic-five-member-ring RGD contained pentapeptides (termed cRGD) were utilized to endow drug vehicles tumor-targeting properties. By combining cyclo(Arg-Gly-Asp-D-Phe-Lys) with PCB-ss-PCL, Huang et al. developed a multifunctional zwitterionic block copolymer cRGD-PCSSL [145]. DOX was encapsulated by the biodegradable PCL segments and the zwitterionic PCB shell would resist nonspecific absorption after intravenous injection to prolong circulation time. Under the guiding of cRGD ligands, the DOX-loading nanoparticles accumulated around cancer cells and would be endocytosed into cells mediated by αvβ3 integrin receptor (Fig. 6). Afterwards, disulfide binds were cut off for redox-responsive drug release.
Fig. 6.
The tumor targeting cRGD-PCSSL micelles guided by cRGDfc peptide. The functional zwitterionic micelles could accumulate around tumor cells leading by cRGD ligands. Through the interaction between cRGD and αvβ3 integrin receptor, the uptake rate of micelles by tumor cells would be enhanced.
On the other hand, since cRGDs are class of pentapeptides, using them to modify zwitterionic polypeptides can achieve unique biomimetic properties as well as tumor targeting function. Given this, Lin and his partners synthesized a cRGD-conjugated polypeptide composed of glutamic acid and lysine to mimic albumin [146]. Because the pair of Glu-Lys has been found as the most abundant AA pairs in albumin, they were chosen for a longer half-life circulation time [147] taking albumin-bound paclitaxel (Abraxane) as a successful precedent [148]. The negative charged DOX was encapsulated in cRGD-poly(L-glutamic acid-co-L-lysine) (cRGD-PMIX) by electrostatic interaction via tuning the ratio of Glu to Lys over 1:1, and the DOX-loaded vesicle was formed through an emulsion solvent evaporation technique. This targeting-functionalized vesicle exhibited pH-sensitive drug release behavior and the existing of trypsin obviously promoted the release of DOX which has been demonstrated to be expressed in kinds of cancers during proliferation, invasion and metastasis [149], [150].
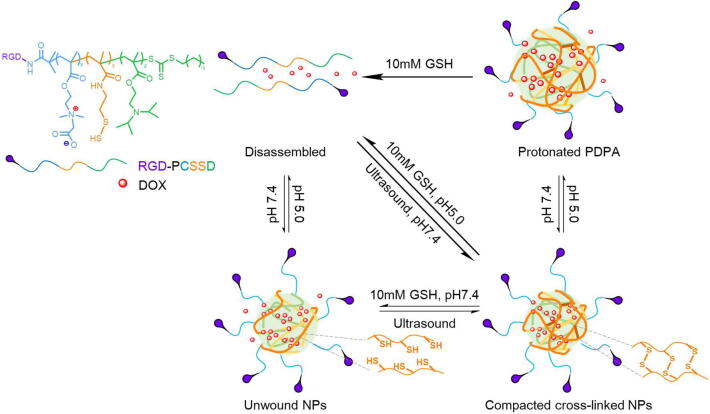
In order to limit drug premature leakage during blood circulation, scientists always take advantage of disulfide bonds to build cross-linked shell. Based on this, Huang et al. designed and synthesized a triblock zwitterionic copolymer that was composed of the disulfide supplier poly(N-(2-(2-pyridyl disulde) ethyl methacrylamide (PDS), the ultra pH-sensitive PDPA core to hold DOX [151] and zwitterionic PCBMA corona to stabilize the nanoparticles and to prolong half-life [152]. The triblock copolymer (PCBMA-PDS-PDPA, called PCSSD) was tumor-targeting functionalized with RGD, so the RGD-PCSSD shell-cross-linked NPs could be guided to tumor sites with negligible biosorption and less premature drug release during systemic circulation. The pH and redox dual-sensitivity of the RGD-PCSSD copolymer was illustrated in Fig. 7. After receptor-mediated endocytosis, the PDPA segment would be protonated to promote escape from endosomes/lysosomes, and the shell linkers would be cut off in respond to GSH with high concentration to facilitate drug release in an intracellular reductive environment.
Fig. 7.
The reduction and pH dual-responsive behavior of the tumor targeting RGD-PCSSD shell-cross-linked nanoparticles. When the pH value was 7.4, the liner RGD-PCSSD copolymers could assemble into NPs using the hydrophobic PDPA core to encapsulate DOX. Treating with ultrasound promoted the formation of disulfide bonds to get compacted cross-linked NPs and held DOX tightly. When the pH value decreased, the PDPA moieties would become protonated which made the NPs swell and a part of drug leak slowly. And as the concentration of GSH in the environment increased, the cross-linked disulfide bonds would be cut off to disrupt the whole system and release the cargoes rapidly.
Hyaluronic acid (HA) is another popular tumor-targeting ligand that performs strong affinity to CD44 which is overexpressed on the surface of many kinds of cancer cells and is associated with tumor progress, invasion and metastasis [153], [154]. Attributed to the carboxyl groups and acetamido groups in HA, Gao et al. prepared a pH-sensitive mix-charged zwitterionic polymer dHAD by deacetylation of HA and grafting dodecylamine onto it [155]. The polymer exhibited negative charge at pH 7.4 and became positive at pH 6.2 which led to an enhanced uptake rate by tumor cells in acidic environment [156]. And since HA is a natural glycosaminoglycan that could be degraded completely by hyaluronidase (HAase) in cells [157], dHAD can provide a choice to form biodegradable DDSs while traditional zwitterionic polymers could not.
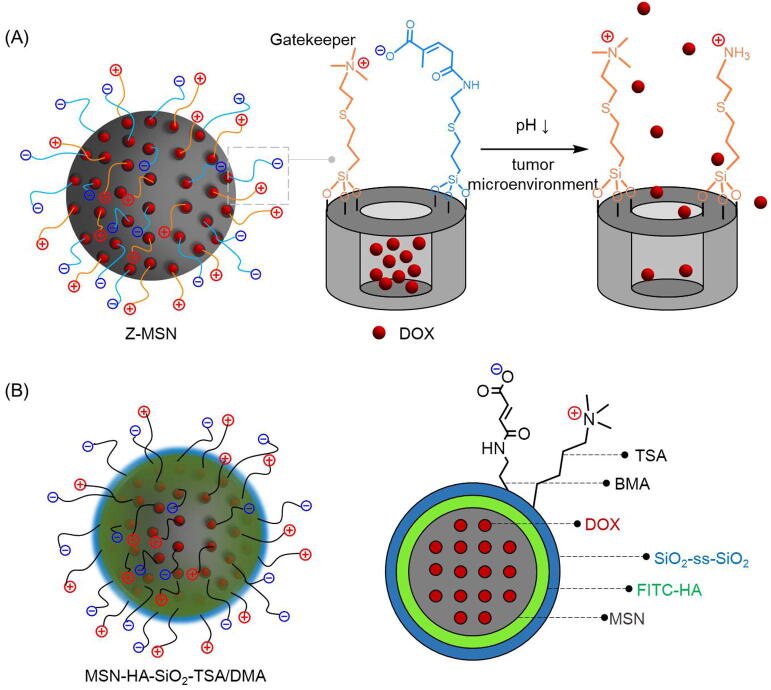
It is interesting to note that Jin et al. developed a triple-layered MSN which used HA as a switch to trigger drug release in response to high concentration HAase, rather than for its tumor targeting [51]. Above the first HA-layer, disulfide bond-embedded silica was utilized as the second GSH-sensitive layer, which was covered by a mix-charged zwitterionic shell (Fig. 8B). This zwitterionic surface consisted of trimethoxysilylpropyl-N,N,N-trimethylammonium chloride (TSA) and 2,3-dimethylmaleic anhydride (DMA), and the net charge of MSN-HA-SiO2-TSA/DMA could switch from neutral to positive in the acidic environment surrounding tumor cells. Mix-charged zwitterions located on the surface of MSN could work as gatekeepers to hold drugs and open the “door” at expected sites (Fig. 8A) [52]. More than that, the HA molecules were labeled by fluorescein isothiocyanate (FITC) which made it possible for real-time drug tracing and selective tumor cell imaging.
Fig. 8.
The “gatekeepers” of Z-MSN and the structural model of MSN-HA-SiO2-TSA/DMA. (A) The carboxylic groups and quaternary amino groups were anchored on the surface of Z-MSN to keep DOX in the caves under the physical condition. And the “doors” would be open after hydrolysis of their carboxylic groups in tumor microenvironment. (B) During the blood circulation, the outermost mix-charged zwitterionic layer could keep away from biomolecules with their dense hydration shell. While the pH decreased from 7.4 to 6.5, the surface charge would shift to cationic hence to promote cellular uptake. After the second GSH-sensitive layer taken off in cytoplasm by GSH with high concentration, the innermost FITC-HA layer would be degraded by HAase to release the therapeutics quickly. And the whole process could be tracked in real-time by fluorescence.
5. Zwitterionic materials applied in gene delivery
As the studies continue persistently, gene therapy has become a predominant strategy for the treatment of genomic and non-genomic diseases [158], [159], [160]. So far, many therapeutic genes are in clinical trials, and some have even been approved for marketing [161], [162]. However, to send the therapeutic genetic molecules to the desired sites and ensure they could transcript and translate into functional proteins successfully, safe and effective carriers are indispensable [163], [164], [165]. The available gene delivery vectors at present generally include viral vectors, non-viral cationic polymers and inorganic mental materials [166], [167], [168]. Although the viral vectors have showed relatively higher gene delivery efficiencies, their immunogenicity makes repeated administration impossible which seriously obstructs the application in clinic. And the risks of insertional mutation as well as difficulties in mass production also limit their application. While cationic polymers can condense negatively charged gene molecules and facilitate cellular uptake of cell membranes, but positively charged macromolecules have considerable cytotoxicity and prefer to interact with other biomolecules in blood [169].
Due to the versatile capabilities, zwitterionic materials also exhibit great potential for gene delivery. The copolymers contain cationic segments and zwitterionic parts are expected as ideal vectors witch inherit the gene-carrying ability of cationic vectors via the interactions with genetic molecules, while the zwitterionic moieties provide a “stealth” surface and stabilize the gene/polymer complexes (polyplexes) sterically [165], [170]. The vinyl-based cationic monomers used to build cationic gene vectors always have a linear/branched structure with monomer unit sequences, such as the derivatives from methacrylamine and methacrylate (shown in Fig. 9) [171]. Most often, zwitterionic molecules are conjugated with these derivatives to form novel gene delivery vectors, and some other types take a zwitterionic coat on the surface of traditional non-viral vectors to endow them with antifouling properties and enhance their stability at meanwhile. Here, the novel gene vectors involved with zwitterionic materials are summarized and displayed in Table 2.
Fig. 9.
The derivatives of methacrylate and methacrylamine are widely used as cationic moieties to build cationic and zwitterionic gene vectors.
Table 2.
Summary of gene vectors associated with the zwitterions.
| Category | Polymers | Loaded gene | Ref. |
|---|---|---|---|
| Phosphorylcholine | PMPC-PDMAEMA | pDNA | [64], [180] |
| PMPC-PDPA | pDNA, siRNA | [65], [66], [67], [181] | |
| PMPC-PAEMA | pDNA | [175] | |
| PMPC-PDMAPAA-PSA | pDNA | [176] | |
| PMDN-HBs | pDNA | [177] | |
| PMPC-PDEA | pDNA | [178], [179] | |
| P((MPC-co-AEMAm)-b-MPC) | pDNA | [183] | |
| Sulfobetaine | PMPDSAH-PDMAEMA | pDNA | [184] |
| PEI-g-PHEAA-b-MEO2MA | pDNA | [185], [186] | |
| PEI-g-PHEAA-b-PMPDSAH | pDNA | [185], [186] | |
| PSBMA-PAA | pDNA | [187] | |
| ZALs | Cas9 mRNA and sgRNA | [190] | |
| MPDSAH-MEO2MA-MPDSAH | pDNA | [197] | |
| Amino acids | OEAL | pDNA | [188] |
| Carboxybetaine | PCB20-DSPE | siRNA | [191], [192] |
| CBAA-PAMAMA | pDNA | [196] | |
| P(CBMA-EE) | pDNA | [198] | |
| PCB-NBE | pDNA | [199] |
5.1. Zwitterion-decorated cationic gene vectors
In the early days, researchers tried to conjugate cationic gene carriers with anionic materials, especially negative charged ligands that are capable of targeting cells to form mix-charged zwitterionic gene carriers. It was reported that conjugating HA to polyethyleneimine (PEI) could reduce the toxicity which was associated with their cationic amine groups, and PEI-HA could promote DNA transfection via the interaction between HA and CD44 on cell surface [172], [173], [174]. However, the DNA condense levels were still decreased with the neutralization of cations. To enhance system stability, limit cytotoxicity but not sacrifice loading capacity, scientists turned to zwitterionic materials for inspiration and innovation.
PC-based cationic polymers have been demonstrated as efficient gene delivery vectors. Ever since the first phospholipid polymer, poly(MPC-2-aminoethyl methacrylate (AEMA)) (PMPC-PAEMA) was developed and applied as a plasmid DNA (pDNA) vector, and quantities of MPC containing polymers were developed for gene delivery [175]. In order to alleviate the non-specific intertwining of vectors and gene molecules, Kitagawa et al. synthesized a battery of micelle-like amphiphilic copolymers, consisting of MPC, N-(3-(dimethylamino)propyl) acrylamide (DMAPAA) and stearyl acrylate (SA) [176]. With the protection of DMAPAA-MPC-SA, the expression level of transfected pCMV-Luc (a plasmid encoding luciferase gene) was proved to be three times higher than that carried by liner-type polycations.
As mentioned in the section of drug delivery system, PMPC-PDPA biblock polymers were utilized as zwitterionic pH-sensitive vectors to load pDNA and siRNA molecules [65], [66], [67]. The MPC could be copolymerized with any vinyl monomer through a common radical polymerization method [177], by replacing the cationic block DPA with other tertiary amine containing monomers, PMPC-PDMAEMA and PMPC-PDEA were copolymerized [64], [178], [179]. Apart from condensing DNA effectively and stabilizing the colloidal size complexes sterically, all of these MPC-based are probable to incorporate with targeting ligands to prepare specific targeting gene vectors. For instance, FA as a frequent-used ligand has been introduced into PMPC-PDMAEMA and PMPC-PDPA diblock copolymers, and the in vitro experiments with FA receptor overexpressing cells [180], [181]. Besides, Chiba et al. copolymerized the poly(MPC-DMAEMA-p-nitrophenylcarbonyloxyethyl methacrylate (NPMA)) (PMDN) as a frame for hepatocyte-specific gene delivery. The NPMA was introduced to immobilize hepatitis B antigen (HBs) so that the PMDN-HBs could transfer therapeutic genes into human hepatocytes specifically [177]. According to the in vivo test, intravenous injection of pDNA/PMDN-HBs did not lead to liver dysfunction or organ weight changes owing to the biocompatibility offered by MPC moieties. More importantly, the specificity of PMDN could be conveniently altered through replacing the liver-targeting HBs with other biorecognition molecules.
Moreover, it was reported that statistical carbohydrate-based copolymers could enhance the expression of loaded genes with low toxicity, but were easier to aggregate in the presence of serum proteins than block copolymers [182]. Therefore, MPC was introduced to form a “block-statistical” copolymer poly(MPC-2-aminoethyl methacrylamide) (PMPC-PAEMAm), exhibiting high gene expression, relatively low-serum protein interaction and low toxicity in hepatocytes and human dermal fibroblasts [183].
Similarly, zwitterionic SB and CB can also perform like PC to improve the cationic gene vectors. As mentioned earlier, the PMPC block could benefit the steric stability of the PDMAEMA/DNA complexes but sacrificed the transfection performance just like the PEGylated cationic vectors. Fortunately, the comb-shaped gene vectors consisted of dextran backbone and diblock copolymeric PCB-PDMAEMA or PSB-PDMAEMA sidechains were demonstrated to work better than PMPC-PDMAEMA, with enhanced DNA transfection as well as high biostability [165]. Liu and co-workers also developed a PSB-cationic methacrylate copolymer poly(N-(3-(methacrylamido)propyl)-N,N-dimethyl-N-(3-sulfopropyl) ammonium hydroxide (MPDSAH)-b-DMAEMA) (PMPDSAH-PDMAEMA) [184], and showed a much higher gene transfection efficiency than PMPC-PDMAEMA, which might by caused by that the hydrophilia of MPC block obstructed the cellular internalization, whereas the SB containing MPDSAH remained the improvement of cellular uptake. But the transfection efficiency of PMPDSAH-PDMAEMA was still lower than cationic PEI (25 k). Since the high molecule weight PEI (25 k) showed high gene transfection ability but high toxicity at the same time, while the low molecule weight (LMW) PEIs were just the reverse, they attempted to graft poly(MEO2MA-b-2-hydroxyethyl methacrylate (HEMA)) (PMEO2MA-PHEMA) and PMPDSAH-PHEAA to LMW PEI (1.2 kDa/1.8 kDa) respectively, and the LMW PEI-g-PHEAA-b-PMPDSAH50 exhibited a high transfection efficiency and stable gene expression level in serum conditions [185], [186].
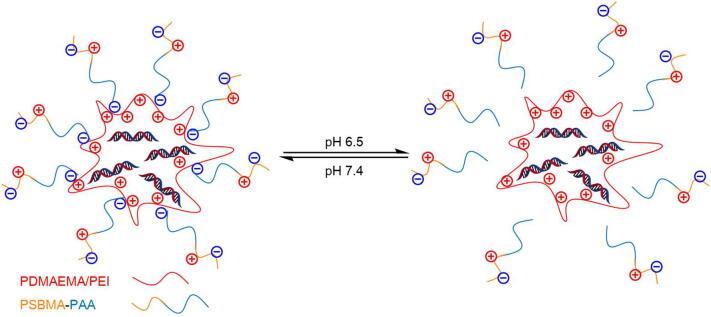
Except for conjugation with cationic polymers, the poly(SBMA-b-acrylic acid (PAA)) (PSBMA-PAA) was employed as a protective shell to PEI/DNA and PDMAEMA/DNA polyplexes (Fig. 10) [187]. Under physiologic conditions, the negative charged PAA could electrostatically interact with the positively charged inner core so that the PSBMA-PAA shell could prevent undesired interactions with blood components which eventually reduced the serum clearance of the therapeutic genes. And the outer shell would be shielded off with the protonation of PAA, when the environmental pH dropt close to the isoelectric point of PAA (pH 6.0) thus to enhance the cellular internalization of DNA.
Fig. 10.
Schematic diagram of PSBMA-PAA/PDMAEMA (or PEI)/DNA core–shell polyplexes. Cationic PDMAEMA (or PEI) first interacted with DNA molecules electrostatically. In physical condition, the diblock polymer PSBMA-PAA served as electroneutral shells to protect the polyplexes from biosorption and fell off around tumor cells to expose the positive charges of the core for enhanced cellular uptake.
Similarly, an amino acid-based pH-responsive charge-conversional copolymer (OEAL) consisting of oligoethylenimine (OEI), PLL and poly(L-aspartate) (PBLA) was prepared to shield PEI/pDNA complexes in a zwitterionic manner [188]. Due to the reversion of shell charges from negative (pH 7.4) to positive (pH 6.8), the entrapped rev-caspase-3 gene could be efficiently transfected into tumor cells and followed by rapidly releasing, and finally induced tumor apoptosis to inhibit cancer progression [189].
What else worth mentioning is that Siegwart et al. managed to design and build the first non-viral carrier for co-delivery of Cas9 mRNAs and sgRNAs [190]. Since the molecular weight of mRNAs is relatively larger, and both the sgRNAs and Cas9 protein must exist in the same individual cell, it is impracticable to use the ordinary gene vectors to deliver the CRISPR/Cas components separately. To overcome the challenges, they synthesized a series of zwitterionic amino lipids (ZALs), containing SB terminal groups and hydrophobic tails with different polyamines as bridges. And the results showed ZALs that held both Cas9 mRNA and sgRNAs could edit target genes effectively with a radio of mRNA: sgRNA ≥ 3:1 (wt).
At meanwhile, zwitterionic PCB-based lipid PCB20-distearoyl phosphoethanolamine (PCB20-DSPE) was developed to modify cationic liposomes for system delivery of therapeutic siRNAs. The zwitterionic PCB20-DSPE could offer stability without influencing the siRNA encapsulation efficiency, or the endosomal/lysosomal escape capability of the lipoplexes [191]. Of course, the PCB modified cationic liposomes could also avoid accelerant blood clearance thus to extend the in vivo circulation time and increase tumor accumulation of therapeutic siRNAs after injection [192]. Recently, PCB-DSPE micelles were also prepared for oral delivery of insulin which were facilitated to penetrate across the epithelial cell layer owing to the biological properties of carboxybetaine [193]. This novel zwitterionic platform might improve the life quality of diabetics who need to receive needle-based injection daily.
Poly(amidoamine) (PAMAM) dendrimer have already been demonstrated to be efficient gene delivery vehicles attributing to their monodispersity, 3D molecular geometry and abundant amine terminal groups. To afford the dendrimers with outstanding compatibility with cells, Wang et al. modified generation 5 (G5) PAMAM dendrimer with carboxybetaine acrylamide (CBAA), which showed barely changes in radius of hydration [194]. And entrapping gold nanoparticles into PAMAM dendrimers has been proved to preserve the 3D spherical shape of dendrimers for better condensation of pDNA and compensate the positive charges of amines partly to alleviate the cytotoxicity [195]. Therefore, Shi et al. first synthesized CBAA and covalently grafted CBAA to the surface of G5 PAMAM dendrimers to entrap gold NPs, and finally got CBAA-functionalized Au DENPs as a vector for the delivery of hypermethylated in cancer 1 (HIC1) pDNA [196]. Because of the abundant terminal amines, PAMAM could condense pDNA strongly and escape from lysosomes via proton sponge effect. And CBAA made Au DENPs display few macrophage cellular uptake to extend their circulation time.
5.2. Gene carriers based on zwitterionic materials
Apart from being used to modify available cationic gene vectors to afford their antifouling abilities, various zwitterionic materials have also participated in therapeutic gene delivery directly.
Since the charge neutralization of cationic polymers tended to cause precipitate of complex after condensing negative charged nucleotide molecules, Dai et al. came up with an idea that synthesizing an double thermo-responsive SB-based “ABA” structured zwitterionic triblock polymers via ATRP, in which “A” represented MPDSAH and “B” was PEG analog MEO2MA with LCST behavior [197]. The SB-based MPDSAH acted to condense DNA molecules via their quaternary ammonium cations, while their sulfonic anions were unreacted thus to prevent from the inter-complex coacervation. Since the positive and negative charges of MPDSAH were separated by three methylene groups, the spatial barrier was weakened so that DNA could be complexed with PMPDSAH effectively. But with the addition of DNA molecules, the ion pairs in MPDSAH would be disrupted which resulted in the disappearance of their UCST, while the LCST still existed with slightly increasement.
In addition, zwitterionic carboxybetaine also shows great potential in gene vector designing thanks to their functional carboxyl groups. Once the COO– groups of PCBs are esterified, the polymers left cationic thus could condense nucleic acid molecules just like the cationic vectors. After ester hydrolysis and exposure of the cationic COO– groups, the gene molecules would be released efficiently. Esterified with the ethyl ester, Jiang et al. developed the CBMA-ethyl ester polymers PCBMAEE with anions hidden by the ester bond partially, so that to render cationic for DNA loading until hydrolysis [198]. They replaced the quaternary ammonium with quaternary (4°)- tertiary (3°)- and secondary (2°)-amine respectively and found a 3:1 mixed copolymer of 3° and 4° CBMA-EE monomers allowed the polyplexes a good size and surface charge to promote transfection and gene expression. On basis of that, they successfully developed a novel photolabile o-nitrobenzyl ester of PCB (PCB-NBE) to endow this platform a UV-sensitive “switch” for active degradation control [199]. Interestingly, in order to maintain the unspecific protein resistance property of PCB, Chen et al. used the polyzwitterionic precursor PCBMAEE as a hydrophobic segment to entrap pDNA as well as guard them against the nuclease attack, and the zwitterionic PCBMA shell was added to improve water-solubility of the core and kept gene form protein in serum [200]. Combining all of the merits, the block polymer PCBMAEE-PCBMA showed high transfection efficiency even to some hard-to-transfect cells like HUVEC, and effective intracellular gene release as well.
6. Summary and outlook
On account of the super hydration ability arising from the ion–dipole interaction among zwitterions and water molecules, zwitterionic materials are gifted with remarkable antifouling property to prevent the adsorption of bacteria, cells and biological macromolecules [15]. And the antifouling property, low cytotoxicity, great biocompatibility, negligible immunogenicity, in vivo stability as well as long circulation time, together make the zwitterions a great choice for drug and therapeutic gene delivery. With the development of multi-functional zwitterionic materials, stimuli-responsive and tumor-targeting ones have been fabricated, which further makes the zwitterions an ideal option for clinical application. Via introducing corporate segments that are protonated in acid environment or pH-sensitive chemical bonds, zwitterionic carriers could respond to environmental pH value to enhance cellular uptake and drug release efficiency; via introducing disulfide bonds which are easily reduced by GSH with high concentration, zwitterionic vectors are gifted redox responsiveness to unload cargoes rapidly in cytoplasm of tumor cells; via utilizing zwitterionic copolymers that are composed of SB-based moieties and adjusting to the fit molar ratios, the SB-decorated delivery systems could be thermo-responsive and transfer from soluble to insoluble hence to release the therapeutics. And the participation of targeting molecules endows zwitterionic carriers with cell and tissue specificity.
Besides, when work as vectors for gene transfection, zwitterions could be used to decorate traditional cationic gene vectors to relieve their cytotoxicity and maintain their ability to hold gene molecules as well as their high cellular uptake efficiency. On the other hand, zwitterionic materials could also serve as gene carriers directly in a charge reversal manner.
Although each type of zwitterions displays outstanding antifouling ability and has been utilized to build therapeutic vectors, there are of course some differences among diverse categories. Since PC is a component of cell membranes, PC-based zwitterions are the earliest to be investigated in this field, and they are also the most ones with relatively thorough study. PC-based biomimetic zwitterionic carriers always show great biocompatibility, but the high synthetic cost might be a fatal problem that limits their application. As for SB-decorated carriers, the most prominent advantage should be the UCST, which is useful to designed thermo-responsive zwitterionic polymers. The UCST is influenced by molar radio and concentration of ions in environment, so adjusting these parameters to fit is necessary. Attributing to the abundant carboxy groups, CB-based vectors are easily functionalized with versatile ligands or biomolecules hence to develop “smart” delivery systems according to demands. And zwitterionic poly(amino acids) or polypeptides are another natural biomimetic materials that can be degraded totally by enzymes through metabolism.
In early days, the patents of zwitterionic materials are mainly about detergent, antifouling coatings and polymer compounds with producing methods thereof. But as the expectations for the safety and healthy become higher, more zwitterionic materials have been granted gradually for DDSs and implant materials in last decades. The classic example PMPC-PDPA have been granted numbers of patents as carriers for drug or genes worldwide, and the patent for siRNA delivery with long-term circulation is going to be published in 2020 (US20200171169A1). The recent trend in relevant patent filings indicates that more and more interests are focused on the development of multi-functional zwitterionic vectors. But while these novel materials have exhibited great potential in the field of biological medicine, studies either on their structures or characters are just in the primary stage, there is still a long way to go to achieve clinical application. One of the most pressing problems is to simplify the synthesis process and reduce the costs which must be faced in the mass production. Further studies are asked to overcome this crucial challenge in the near future. Meanwhile, with the increasing demands for precise control of drug location and release, the application of multiple stimuli-responsive DDSs is an irresistible trend, which can not only send the drugs to desired destinations with few premature leakage, but also unload them at the exact sites (even at the expected cell-organelle) to limit side effects and ensure the therapeutic effect. In addition, since the quantity of zwitterionic gene vectors at present is obviously less than carriers for drugs, it might suggest a much huger development space and brighter future for versatile zwitterionic materials utilized for therapeutic genes’ delivery. From this prospective, more attention might be paid to enhance the transfection efficiency of therapeutic genes with the help of zwitterionic delivery systems. All in all, in order to achieve clinical application, more investigations are needed to improve zwitterionic materials’ performance in biocompatibility, biodegradability, drug loading capacity, low cytotoxicity, environmental stimuli-responsibility, specific targeting ability and some other peculiarities in light of requirements.
CRediT authorship contribution statement
Ling-Yan Zhou: Data curation, Formal analysis, Writing - review & editing. Yang-Hui Zhu: Data curation, Writing - original draft, Software. Xiao-Yu Wang: Investigation, Methodology. Chao Shen: Methodology. Xia-Wei Wei: Supervision. Ting Xu: Conceptualization, Funding acquisition, Project administration, Supervision. Zhi-Yao He: Conceptualization, Funding acquisition, Methodology, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant number 81602699); the National High-Tech R&D Program of China (grant number 2015AA020309); the Sichuan Science and Technology program (grant numbers 2018GZ0311, 2019YFG0266); and the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (grant number ZYJC18028).
Contributor Information
Ting Xu, Email: tingx2009@163.com.
Zhi-Yao He, Email: zhiyaohe@scu.edu.cn.
References
- 1.Frokjaer S., Otzen D.E. Protein drug stability: a formulation challenge. Nat Rev Drug Discov. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 2.Otsuka H., Nagasaki Y., Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2003;55:403–419. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 3.Shuai X., Ai H., Nasongkla N., Kim S., Gao J. Micellar carriers based on block copolymers of poly(epsilon-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J Control Release. 2004;98:415–426. doi: 10.1016/j.jconrel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z., Xiong X., Wan J., Xiao L., Gan L. Cellular uptake and intracellular trafficking of PEG-b-PLA polymeric micelles. Biomaterials. 2012;33:7233–7240. doi: 10.1016/j.biomaterials.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 5.Gaberc-Porekar V., Zore I., Podobnik B., Menart V. Obstacles and pitfalls in the PEGylation of therapeutic proteins. Curr Opin Drug Discov Devel. 2008;11:242–250. [PubMed] [Google Scholar]
- 6.Shen M., Martinson L., Wagner M.S., Castner D.G., Ratner B.D. PEO-like plasma polymerized tetraglyme surface interactions with leukocytes and proteins: in vitro and in vivo studies. J Biomater Sci Polym Ed. 2002;13:367–390. doi: 10.1163/156856202320253910. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Cao Z., Bai T., Carr L., Ella-Menye J.R. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat Biotechnol. 2013;31:553–556. doi: 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]
- 8.Hatakeyama H., Akita H., Ito E., Hayashi Y., Oishi M. Systemic delivery of siRNA to tumors using a lipid nanoparticle containing a tumor-specific cleavable PEG-lipid. Biomaterials. 2011;32:4306–4316. doi: 10.1016/j.biomaterials.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 9.He Z.Y., Chu B.Y., Wei X.W., Li J., Edwards C.K.I. Recent development of poly(ethylene glycol)-cholesterol conjugates as drug delivery systems. Int J Pharm. 2014;469:168–178. doi: 10.1016/j.ijpharm.2014.04.056. [DOI] [PubMed] [Google Scholar]
- 10.He Z.Y., Shi K., Wei Y.Q., Qian Z.Y. Recent advances of poly(ether-ether) and poly(ether-ester) block copolymers in biomedical applications. Curr Drug Metab. 2016;17:168–186. doi: 10.2174/1389200216666151103115944. [DOI] [PubMed] [Google Scholar]
- 11.He M., Gao K., Zhou L., Jiao Z., Wu M. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016;40:142–152. doi: 10.1016/j.actbio.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Shao Q., Jiang S. Molecular understanding and design of zwitterionic materials. Adv Mater. 2015;27:15–26. doi: 10.1002/adma.201404059. [DOI] [PubMed] [Google Scholar]
- 13.Zou H., Wang Z., Feng M. Nanocarriers with tunable surface properties to unblock bottlenecks in systemic drug and gene delivery. J Control Release. 2015;214:121–133. doi: 10.1016/j.jconrel.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 14.He Y., Hower J., Chen S., Bernards M.T., Chang Y. Molecular simulation studies of protein interactions with zwitterionic phosphorylcholine self-assembled monolayers in the presence of water. Langmuir. 2008;24:10358–10364. doi: 10.1021/la8013046. [DOI] [PubMed] [Google Scholar]
- 15.Schlenoff J.B. Zwitteration: coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir. 2014;30:9625–9636. doi: 10.1021/la500057j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee I., Pangule R.C., Kane R.S. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv Mater. 2011;23:690–718. doi: 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- 17.Pombo Garcia K., Zarschler K., Barbaro L., Barreto J.A., O'Malley W. Zwitterionic-coated “stealth” nanoparticles for biomedical applications: recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small. 2014;10:2516–2529. doi: 10.1002/smll.201303540. [DOI] [PubMed] [Google Scholar]
- 18.Schottler S., Landfester K., Mailander V. Controlling the stealth effect of nanocarriers through understanding the protein corona. Angew Chem Int Ed Engl. 2016;55:8806–8815. doi: 10.1002/anie.201602233. [DOI] [PubMed] [Google Scholar]
- 19.Leng C., Sun S., Zhang K., Jiang S., Chen Z. Molecular level studies on interfacial hydration of zwitterionic and other antifouling polymers in situ. Acta Biomater. 2016;40:6–15. doi: 10.1016/j.actbio.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Ostuni E., Chapman R.G., Holmlin R.E., Takayama S., Whitesides G.M. A survey of structure-property relationships of surfaces that resist the adsorption of protein. Langmuir. 2001;17:5605–5620. doi: 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- 21.Kainthan R.K., Brooks D.E. In vivo biological evaluation of high molecular weight hyperbranched polyglycerols. Biomaterials. 2007;28:4779–4787. doi: 10.1016/j.biomaterials.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 22.Gillich T., Acikgoz C., Isa L., Schluter A.D., Spencer N.D. PEG-stabilized core-shell nanoparticles: impact of linear versus dendritic polymer shell architecture on colloidal properties and the reversibility of temperature-induced aggregation. ACS Nano. 2013;7:316–329. doi: 10.1021/nn304045q. [DOI] [PubMed] [Google Scholar]
- 23.Faulon Marruecos D., Kastantin M., Schwartz D.K., Kaar J.L. Dense poly(ethylene glycol) brushes reduce adsorption and stabilize the unfolded conformation of fibronectin. Biomacromolecules. 2016;17:1017–1025. doi: 10.1021/acs.biomac.5b01657. [DOI] [PubMed] [Google Scholar]
- 24.Gal N., Schroffenegger M., Reimhult E. Stealth nanoparticles grafted with dense polymer brushes display adsorption of serum protein investigated by isothermal titration calorimetry. J Phys Chem B. 2018;122:5820–5834. doi: 10.1021/acs.jpcb.8b02338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelaz B., del Pino P., Maffre P., Hartmann R., Gallego M. Surface functionalization of nanoparticles with polyethylene glycol: effects on protein adsorption and cellular uptake. ACS Nano. 2015;9:6996–7008. doi: 10.1021/acsnano.5b01326. [DOI] [PubMed] [Google Scholar]
- 26.Leng C., Hung H.C., Sun S., Wang D., Li Y. Probing the surface hydration of nonfouling zwitterionic and PEG materials in contact with proteins. ACS Appl Mater Interfaces. 2015;7:16881–16888. doi: 10.1021/acsami.5b05627. [DOI] [PubMed] [Google Scholar]
- 27.Chen H., Yang J., Xiao S., Hu R., Bhaway S.M. Salt-responsive polyzwitterionic materials for surface regeneration between switchable fouling and antifouling properties. Acta Biomater. 2016;40:62–69. doi: 10.1016/j.actbio.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Yang J., Chen H., Xiao S., Shen M., Chen F. Salt-responsive zwitterionic polymer brushes with tunable friction and antifouling properties. Langmuir. 2015;31:9125–9133. doi: 10.1021/acs.langmuir.5b02119. [DOI] [PubMed] [Google Scholar]
- 29.Das S., Banik M., Chen G., Sinha S., Mukherjee R. Polyelectrolyte brushes: theory, modelling, synthesis and applications. Soft Matter. 2015;11:8550–8583. doi: 10.1039/c5sm01962a. [DOI] [PubMed] [Google Scholar]
- 30.Iwasaki Y., Ishihara K. Cell membrane-inspired phospholipid polymers for developing medical devices with excellent biointerfaces. Sci Technol Adv Mater. 2012;13:064101. doi: 10.1088/1468-6996/13/6/064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Son S., Kim G., Singha K., Park S., Ree M. Artificial cell membrane-mimicking nanostructure facilitates efficient gene delivery through fusogenic interaction with the plasma membrane of living cells. Small. 2011;7:2991–2997. doi: 10.1002/smll.201100232. [DOI] [PubMed] [Google Scholar]
- 32.Ishihara K., Ueda T., Nakabayashi N. reparation of phospholipid polylners and their properties as polymer hydrogel membranes. Polym J. 1990;22:355–360. [Google Scholar]
- 33.Konno T., Watanabe J., Ishihara K. Enhanced solubility of paclitaxel using water-soluble and biocompatible 2-methacryloyloxyethyl phosphorylcholine polymers. J Biomed Mater Res A. 2003;65:209–214. doi: 10.1002/jbm.a.10481. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J., Chai Y.D., Zhang J., Huang P.F., Nakashima K. Long circulating micelles of an amphiphilic random copolymer bearing cell outer membrane phosphorylcholine zwitterions. Acta Biomater. 2015;16:94–102. doi: 10.1016/j.actbio.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 35.Ueda T., Oshida H., Kurita K., Ishihara K., Nakabayashi N. Preparation of 2-methacryloyloxyethyl phosphorylcholine copolymers with alkyl methacrylates and their blood compatibility. Polym J. 1992;24:1259–1269. [Google Scholar]
- 36.Xu J.P., Ji J., Chen W.D., Shen J.C. Novel biomimetic polymersomes as polymer therapeutics for drug delivery. J Control Release. 2005;107:502–512. doi: 10.1016/j.jconrel.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Lowe A.B., McCormick C.L. Synthesis and solution properties of zwitterionic polymers. Chem Rev. 2002;102:4177–4189. doi: 10.1021/cr020371t. [DOI] [PubMed] [Google Scholar]
- 38.Wu J., Lin W.L., Wang Z. Investigation of the hydration of nonfouling material poly(sulfobetaine methacrylate) by low-field nuclear magnetic resonance. Langmuir. 2012;28:7436–7441. doi: 10.1021/la300394c. [DOI] [PubMed] [Google Scholar]
- 39.Ye L., Zhang Y., Yang B., Zhou X., Li J. Zwitterionic-modified starch-based stealth micelles for prolonging circulation time and reducing macrophage response. ACS Appl Mater Interfaces. 2016;8:4385–4398. doi: 10.1021/acsami.5b10811. [DOI] [PubMed] [Google Scholar]
- 40.Chang Y., Yandi W., Chen W.Y., Shih Y.J., Yang C.C. Tunable bioadhesive copolymer hydrogels of thermoresponsive poly(N-isopropyl acrylamide) containing zwitterionic polysulfobetaine. Biomacromolecules. 2010;11:1101–1110. doi: 10.1021/bm100093g. [DOI] [PubMed] [Google Scholar]
- 41.Shao Q., Mi L., Han X., Bai T., Liu S. Differences in cationic and anionic charge densities dictate zwitterionic associations and stimuli responses. J Phys Chem B. 2014;118:6956–6962. doi: 10.1021/jp503473u. [DOI] [PubMed] [Google Scholar]
- 42.Dong Z.X., Mao J., Yang M.Q., Wang D.P., Bo S.Q. Phase behavior of poly(sulfobetaine methacrylate)-grafted silica nanoparticles and their stability in protein solutions. Langmuir. 2011;27:15282–15291. doi: 10.1021/la2038558. [DOI] [PubMed] [Google Scholar]
- 43.Liu L.D., Wang T., Liu C., Lin K., Liu G.M. Specific anion effect in water-nonaqueous solvent mixtures: interplay of the interactions between anion, solvent, and polymer. J Phys Chem B. 2013;117:10936–10943. doi: 10.1021/jp406215c. [DOI] [PubMed] [Google Scholar]
- 44.Tian M., Wang J.M., Zhang E.S., Li J.J., Duan C.M. Synthesis of agarose-graft-poly[3-dimethyl (methacryloyloxyethyl) ammonium propanesulfonate] zwitterionic graft copolymers via ATRP and their thermally-induced aggregation behavior in aqueous media. Langmuir. 2013;29:8076–8085. doi: 10.1021/la4007668. [DOI] [PubMed] [Google Scholar]
- 45.Yang B.G., Wang C.Y., Zhang Y.B., Ye L., Qian Y.F. A thermoresponsive poly(N-vinylcaprolactam-co-sulfobetaine methacrylate) zwitterionic hydrogel exhibiting switchable anti-biofouling and cytocompatibility. Polym Chem. 2015;6:3431–3442. [Google Scholar]
- 46.Zhang Z., Chen S., Jiang S. Dual-functional biomimetic materials: nonfouling poly(carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules. 2006;7:3311–3315. doi: 10.1021/bm060750m. [DOI] [PubMed] [Google Scholar]
- 47.Jiang L., Zhu C., Fu Y., Yang G. Amino acid functionalization of doped single-walled carbon nanotubes: effects of dopants and side chains as well as zwitterionic stabilizations. J Phys Chem B. 2017;121:2721–2730. doi: 10.1021/acs.jpcb.6b12199. [DOI] [PubMed] [Google Scholar]
- 48.Banskota S., Yousefpour P., Kirmani N., Li X., Chilkoti A. Long circulating genetically encoded intrinsically disordered zwitterionic polypeptides for drug delivery. Biomaterials. 2019;192:475–485. doi: 10.1016/j.biomaterials.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang S., Cao Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv Mater. 2010;22:920–932. doi: 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]
- 50.Qin Z., Chen T., Teng W., Jin Q., Ji J. Mixed-charged zwitterionic polymeric micelles for tumor acidic environment responsive intracellular drug delivery. Langmuir. 2019;35:1242–1248. doi: 10.1021/acs.langmuir.8b00471. [DOI] [PubMed] [Google Scholar]
- 51.Jin R., Liu Z., Bai Y., Zhou Y., Chen X. Multiple-responsive mesoporous silica nanoparticles for highly accurate drugs delivery to tumor cells. ACS Omega. 2018;3:4306–4315. doi: 10.1021/acsomega.8b00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khatoon S., Han H.S., Lee M., Lee H., Jung D.W. Zwitterionic mesoporous nanoparticles with a bioresponsive gatekeeper for cancer therapy. Acta Biomater. 2016;40:282–292. doi: 10.1016/j.actbio.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Mi P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics. 2020;10:4557–4588. doi: 10.7150/thno.38069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boudier A., Aubert-Pouessel A., Louis-Plence P., Gerardin C., Jorgensen C. The control of dendritic cell maturation by pH-sensitive polyion complex micelles. Biomaterials. 2009;30:233–241. doi: 10.1016/j.biomaterials.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 55.Deirram N., Zhang C., Kermaniyan S.S., Johnston A.P.R., Such G.K. pH-Responsive polymer nanoparticles for drug delivery. Macromol Rapid Commun. 2019;40:e1800917. doi: 10.1002/marc.201800917. [DOI] [PubMed] [Google Scholar]
- 56.Ulbrich K., Subr V. Polymeric anticancer drugs with pH-controlled activation. Adv Drug Deliv Rev. 2004;56:1023–1050. doi: 10.1016/j.addr.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 57.Such G.K., Yan Y., Johnston A.P.R., Gunawan S.T., Caruso F. Interfacing materials science and biology for drug carrier design. Adv Mater. 2015;27:2278–2297. doi: 10.1002/adma.201405084. [DOI] [PubMed] [Google Scholar]
- 58.Yang Q., Wang S.H., Fan P.W., Wang L.F., Di Y. pH-responsive carrier system based on carboxylic acid modified mesoporous silica and polyelectrolyte for drug delivery. Chem Mater. 2005;17:5999–6003. [Google Scholar]
- 59.Selby LI, Cortez-Jugo CM, Such GK, Johnston APR. Nanoescapology: progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology 9, 2017. [DOI] [PubMed]
- 60.Massignani M., LoPresti C., Blanazs A., Madsen J., Armes S.P. Controlling cellular uptake by surface chemistry, size, and surface topology at the nanoscale. Small. 2009;5:2424–2432. doi: 10.1002/smll.200900578. [DOI] [PubMed] [Google Scholar]
- 61.Giacomelli C., Le Men L., Borsali R., Lai-Kee-Him J., Brisson A. Phosphorylcholine-based pH-responsive diblock copolymer micelles as drug delivery vehicles: light scattering, electron microscopy, and fluorescence experiments. Biomacromolecules. 2006;7:817–828. doi: 10.1021/bm0508921. [DOI] [PubMed] [Google Scholar]
- 62.Colley H.E., Hearnden V., Avila-Olias M., Cecchin D., Canton I. Polymersome-mediated delivery of combination anticancer therapy to head and neck cancer cells: 2D and 3D in vitro evaluation. Mol Pharm. 2014;11:1176–1188. doi: 10.1021/mp400610b. [DOI] [PubMed] [Google Scholar]
- 63.Pegoraro C., Cecchin D., Gracia L.S., Warren N., Madsen J. Enhanced drug delivery to melanoma cells using PMPC-PDPA polymersomes. Cancer Lett. 2013;334:328–337. doi: 10.1016/j.canlet.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 64.Zhao X., Zhang Z., Pan F., Waigh T.A., Lu J.R. Plasmid DNA complexation with phosphorylcholine diblock copolymers and its effect on cell transfection. Langmuir. 2008;24:6881–6888. doi: 10.1021/la800593q. [DOI] [PubMed] [Google Scholar]
- 65.Lomas H., Du J., Canton I., Madsen J., Warren N. Efficient encapsulation of plasmid DNA in pH-sensitive PMPC-PDPA polymersomes: study of the effect of PDPA block length on copolymer-DNA binding affinity. Macromol Biosci. 2010;10:513–530. doi: 10.1002/mabi.201000083. [DOI] [PubMed] [Google Scholar]
- 66.Du J., Tang Y., Lewis A.L., Armes S.P. pH-sensitive vesicles based on a biocompatible zwitterionic diblock copolymer. J Am Chem Soc. 2005;127:17982–17983. doi: 10.1021/ja056514l. [DOI] [PubMed] [Google Scholar]
- 67.Yu H., Zou Y., Jiang L., Yin Q., He X. Induction of apoptosis in non-small cell lung cancer by downregulation of MDM2 using pH-responsive PMPC-b-PDPA/siRNA complex nanoparticles. Biomaterials. 2013;34:2738–2747. doi: 10.1016/j.biomaterials.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 68.Zhai S.Y., Ma Y.H., Chen Y.Y., Li D., Cao J. Synthesis of an amphiphilic block copolymer containing zwitterionic sulfobetaine as a novel pH-sensitive drug carrier. Polym Chem. 2014;5:1285–1297. [Google Scholar]
- 69.Abbasi M.A., Hassan M., Ur-Rehman A., Siddiqui S.Z., Hussain G. 2-Furoic piperazide derivatives as promising drug candidates of type 2 diabetes and Alzheimer's diseases: in vitro and in silico studies. Comput Biol Chem. 2018;77:72–86. doi: 10.1016/j.compbiolchem.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 70.Huang X., Xiao Y., Lang M. Self-assembly of pH-sensitive mixed micelles based on linear and star copolymers for drug delivery. J Colloid Interface Sci. 2011;364:92–99. doi: 10.1016/j.jcis.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 71.Park J.S., Han T.H., Lee K.Y., Han S.S., Hwang J.J. N-acetyl histidine-conjugated glycol chitosan self-assembled nanoparticles for intracytoplasmic delivery of drugs: endocytosis, exocytosis and drug release. J Control Release. 2006;115:37–45. doi: 10.1016/j.jconrel.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 72.Bayoumy A., El-Sayed E.S.M., Yousef Omar A., Ibrahim M. Emerging applications of chitosan: from biology to environment. Biointer Res Appl Chem. 2018;8:3368–3380. [Google Scholar]
- 73.Wu M., Cao Z., Zhao Y., Zeng R., Tu M. Novel self-assembled pH-responsive biomimetic nanocarriers for drug delivery. Mater Sci Eng C Mater Biol Appl. 2016;64:346–353. doi: 10.1016/j.msec.2016.03.099. [DOI] [PubMed] [Google Scholar]
- 74.Obata Y., Tajima S., Takeoka S. Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo. J Control Release. 2010;142:267–276. doi: 10.1016/j.jconrel.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 75.Mo R., Sun Q., Li N., Zhang C. Intracellular delivery and antitumor effects of pH-sensitive liposomes based on zwitterionic oligopeptide lipids. Biomaterials. 2013;34:2773–2786. doi: 10.1016/j.biomaterials.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 76.Wu L., Ni C., Zhang L., Shi G. Preparation of pH-sensitive zwitterionic nano micelles and drug controlled release for enhancing cellular uptake. J Biomater Sci Polym Ed. 2016;27:643–656. doi: 10.1080/09205063.2016.1147797. [DOI] [PubMed] [Google Scholar]
- 77.Mo R., Sun Q., Xue J., Li N., Li W. Multistage pH-responsive liposomes for mitochondrial-targeted anticancer drug delivery. Adv Mater. 2012;24:3659–3665. doi: 10.1002/adma.201201498. [DOI] [PubMed] [Google Scholar]
- 78.Lee M.J., Ye A.S., Gardino A.K., Heijink A.M., Sorger P.K. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149:780–794. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He Y., Su Z., Xue L., Xu H., Zhang C. Co-delivery of erlotinib and doxorubicin by pH-sensitive charge conversion nanocarrier for synergistic therapy. J Control Release. 2016;229:80–92. doi: 10.1016/j.jconrel.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 80.Vallet-Regi M., Colilla M., Izquierdo-Barba I., Manzano M. Mesoporous silica nanoparticles for drug delivery: current insights. Molecules. 2017;23:47. doi: 10.3390/molecules23010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Encinas N., Angulo M., Astorga C., Colilla M., Izquierdo-Barba I. Mixed-charge pseudo-zwitterionic mesoporous silica nanoparticles with low-fouling and reduced cell uptake properties. Acta Biomater. 2019;84:317–327. doi: 10.1016/j.actbio.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bae Y., Kataoka K. Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv Drug Deliv Rev. 2009;61:768–784. doi: 10.1016/j.addr.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 83.Wang H.B., Liu X.S., Wang Y., Chen Y.J., Jin Q. Doxorubicin conjugated phospholipid prodrugs as smart nanomedicine platforms for cancer therapy. J Mater Chem B. 2015;3:3297–3305. doi: 10.1039/c4tb01984a. [DOI] [PubMed] [Google Scholar]
- 84.Hsiue G.H., Lo C.L., Cheng C.H., Lin C.P., Huang C.K. Preparation and characterization of poly (2-methacryloyloxyethyl phosphorylcholine)-block-poly(D, L-lactide) polymer nanoparticles. J Polym Sci Pol Chem. 2007;45:688–698. [Google Scholar]
- 85.Liu G.Y., Lv L.P., Chen C.J., Liu X.S., Hu X.F. Biocompatible and biodegradable polymersomes for pH-triggered drug release. Soft Matter. 2011;7:6629–6636. [Google Scholar]
- 86.Ma B., Zhuang W., Liu G., Wang Y. A biomimetic and pH-sensitive polymeric micelle as carrier for paclitaxel delivery. Regen Biomater. 2018;5:15–24. doi: 10.1093/rb/rbx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma B., Zhuang W., Wang Y., Luo R., Wang Y. pH-sensitive doxorubicin-conjugated prodrug micelles with charge-conversion for cancer therapy. Acta Biomater. 2018;70:186–196. doi: 10.1016/j.actbio.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 88.Khunsuk P.O., Chawalitpong S., Sawutdeechaikul P., Palaga T., Hoven V.P. Gold nanorods stabilized by biocompatible and multifunctional zwitterionic copolymer for synergistic cancer therapy. Mol Pharm. 2018;15:164–174. doi: 10.1021/acs.molpharmaceut.7b00780. [DOI] [PubMed] [Google Scholar]
- 89.Hou W., Zhao X., Qian X., Pan F., Zhang C. pH-Sensitive self-assembling nanoparticles for tumor near-infrared fluorescence imaging and chemo-photodynamic combination therapy. Nanoscale. 2016;8:104–116. doi: 10.1039/c5nr06842h. [DOI] [PubMed] [Google Scholar]
- 90.Chen X., Parelkar S.S., Henchey E., Schneider S., Emrick T. PolyMPC-doxorubicin prodrugs. Bioconjug Chem. 2012;23:1753–1763. doi: 10.1021/bc200667s. [DOI] [PubMed] [Google Scholar]
- 91.Du J.Z., Du X.J., Mao C.Q., Wang J. Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J Am Chem Soc. 2011;133:17560–17563. doi: 10.1021/ja207150n. [DOI] [PubMed] [Google Scholar]
- 92.Liu N., Han J., Zhang X., Yang Y., Liu Y. pH-responsive zwitterionic polypeptide as a platform for anti-tumor drug delivery. Colloids Surf B Biointerfaces. 2016;145:401–409. doi: 10.1016/j.colsurfb.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y., Huang D., Wang X., Yang F., Shen H. Fabrication of zwitterionic and pH-responsive polyacetal dendrimers for anticancer drug delivery. Biomater Sci. 2019;7:3238–3248. doi: 10.1039/c9bm00606k. [DOI] [PubMed] [Google Scholar]
- 94.Caminade A.M., Turrin C.O. Dendrimers for drug delivery. J Mater Chem B. 2014;2:4055–4066. doi: 10.1039/c4tb00171k. [DOI] [PubMed] [Google Scholar]
- 95.Huang D., Zhuang Y., Shen H., Yang F., Wang X. Acetal-linked PEGylated paclitaxel prodrugs forming free-paclitaxel-loaded pH-responsive micelles with high drug loading capacity and improved drug delivery. Mater Sci Eng C Mater Biol Appl. 2018;82:60–68. doi: 10.1016/j.msec.2017.08.063. [DOI] [PubMed] [Google Scholar]
- 96.Xiao L., Huang L., Moingeon F., Gauthier M., Yang G. pH-responsive poly(ethylene glycol)-block-polylactide micelles for tumor-targeted drug delivery. Biomacromolecules. 2017;18:2711–2722. doi: 10.1021/acs.biomac.7b00509. [DOI] [PubMed] [Google Scholar]
- 97.You Y.Z., Hong C.Y., Pan C.Y. Facile one-pot approach for preparing dually responsive core-shell nanostructure. Macromolecules. 2009:573–575. [Google Scholar]
- 98.Alvarez-Lorenzo C., Bromberg L., Concheiro A. Light-sensitive intelligent drug delivery systems. Photochem Photobiol. 2009;85:848–860. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 99.Fang Z., Pan S., Gao P., Sheng H., Li L. Stimuli-responsive charge-reversal nano drug delivery system: the promising targeted carriers for tumor therapy. Int J Pharm. 2020;575:118841. doi: 10.1016/j.ijpharm.2019.118841. [DOI] [PubMed] [Google Scholar]
- 100.Cai G., Zhang H., Liu P., Wang L., Jiang H. Triggered disassembly of hierarchically assembled onion-like micelles into the pristine core-shell micelles via a small change in pH. Acta Biomater. 2011;7:3729–3737. doi: 10.1016/j.actbio.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 101.Zhao W., Hu J., Gao W. Glucose oxidase-polymer nanogels for synergistic cancer-starving and oxidation therapy. ACS Appl Mater Interfaces. 2017;9:23528–23535. doi: 10.1021/acsami.7b06814. [DOI] [PubMed] [Google Scholar]
- 102.Yang X., Cai X., Yu A., Xi Y., Zhai G. Redox-sensitive self-assembled nanoparticles based on alpha-tocopherol succinate-modified heparin for intracellular delivery of paclitaxel. J Colloid Interface Sci. 2017;496:311–326. doi: 10.1016/j.jcis.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 103.Lee M.H., Yang Z., Lim C.W., Lee Y.H., Dongbang S. Disulfide-cleavage-triggered chemosensors and their biological applications. Chem Rev. 2013;113:5071–5109. doi: 10.1021/cr300358b. [DOI] [PubMed] [Google Scholar]
- 104.Cai M., Cao J., Wu Z., Cheng F., Chen Y. In vitro and in vivo anti-tumor efficiency comparison of phosphorylcholine micelles with PEG micelles. Colloids Surf B Biointerfaces. 2017;157:268–279. doi: 10.1016/j.colsurfb.2017.05.053. [DOI] [PubMed] [Google Scholar]
- 105.Tu S., Chen Y.W., Qiu Y.B., Zhu K., Luo X.L. Enhancement of cellular uptake and antitumor efficiencies of micelles with phosphorylcholine. Macromol Biosci. 2011;11:1416–1425. doi: 10.1002/mabi.201100111. [DOI] [PubMed] [Google Scholar]
- 106.Cai M., Wu Z., Li Y., Cao J., Chen Y. Bioinspired mimics: self-assembly of redox-activated phosphorylcholine-based biodegradable copolymers for enhancing antitumor efficiency. Mater Sci Eng C Mater Biol Appl. 2018;89:401–412. doi: 10.1016/j.msec.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 107.Sun H.L., Guo B.N., Li X.Q., Cheng R., Meng F.H. Shell-sheddable micelles based on dextran-SS-poly(epsilon-caprolactone) diblock copolymer for efficient intracellular release of doxorubicin. Biomacromolecules. 2010;11:848–854. doi: 10.1021/bm1001069. [DOI] [PubMed] [Google Scholar]
- 108.Jiang J., Li J., Zhou B., Niu C., Wang W. Fabrication of polymer micelles with zwitterionic shell and biodegradable core for reductively responsive release of doxorubicin. Polymers (Basel) 2019;11:1019. doi: 10.3390/polym11061019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dai J., Lin S.D., Cheng D., Zou S.Y., Shuai X.T. Interlayer-crosslinked micelle with partially hydrated core showing reduction and pH dual sensitivity for pinpointed intracellular drug release. Angew Chem Int Edit. 2011;50:9404–9408. doi: 10.1002/anie.201103806. [DOI] [PubMed] [Google Scholar]
- 110.Men Y., Peng S., Yang P., Jiang Q., Zhang Y. Biodegradable zwitterionic nanogels with long circulation for antitumor drug delivery. ACS Appl Mater Inter. 2018;10:23509–23521. doi: 10.1021/acsami.8b03943. [DOI] [PubMed] [Google Scholar]
- 111.Lin W., He Y., Zhang J., Wang L., Wang Z. Highly hemocompatible zwitterionic micelles stabilized by reversible cross-linkage for anti-cancer drug delivery. Colloids Surf B Biointerfaces. 2014;115:384–390. doi: 10.1016/j.colsurfb.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 112.Liu P., Shi B.H., Yue C.X., Gao G.H., Li P. Dextran-based redox-responsive doxorubicin prodrug micelles for overcoming multidrug resistance. Poly Chem. 2013;4:5793–5799. [Google Scholar]
- 113.Hu X.L., Hu J.M., Tian J., Ge Z.S., Zhang G.Y. Polyprodrug amphiphiles: hierarchical assemblies for shape-regulated cellular internalization, trafficking, and drug delivery. J Am Chem Soc. 2013;135:17617–17629. doi: 10.1021/ja409686x. [DOI] [PubMed] [Google Scholar]
- 114.Gharbavi M., Amani J., Kheiri-Manjili H., Danafar H., Sharafi A. Niosome: a promising nanocarrier for natural drug delivery through blood-brain barrier. Adv Pharmacol Sci. 2018;2018:6847971. doi: 10.1155/2018/6847971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ward S.M., Skinner M., Saha B., Emrick T. Polymer-temozolomide conjugates as therapeutics for treating glioblastoma. Mol Pharm. 2018;15:5263–5276. doi: 10.1021/acs.molpharmaceut.8b00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu D., Tang J., Shen Y. Zwitterionic poly(lysine methacrylate) brush as an effective carrier for drug delivery. J Control Release. 2015;213:e27–e28. doi: 10.1016/j.jconrel.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 117.Li S.D., Huang L. Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mol Pharmaceut. 2006;3:579–588. doi: 10.1021/mp060039w. [DOI] [PubMed] [Google Scholar]
- 118.Hu J., Xu Y., Zhang Y. Amphiphilic random polycarbonate self-assemble into GSH/pH dual responsive micelle-like aggregates in water. Chin Chem Lett. 2019;30:2039–2042. [Google Scholar]
- 119.Cai M., Leng M., Lu A., He L., Xie X. Synthesis of amphiphilic copolymers containing zwitterionic sulfobetaine as pH and redox responsive drug carriers. Colloids Surf B Biointerfaces. 2015;126:1–9. doi: 10.1016/j.colsurfb.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 120.Ma J., Kang K., Zhang Y., Yi Q., Gu Z. Detachable polyzwitterion-coated ternary nanoparticles based on peptide dendritic carbon dots for efficient drug delivery in cancer therapy. ACS Appl Mater Interfaces. 2018;10:43923–43935. doi: 10.1021/acsami.8b17041. [DOI] [PubMed] [Google Scholar]
- 121.Othman B.A., Greenwood C., Abuelela A.F., Bharath A.A., Chen S. Correlative light-electron microscopy shows RGD-targeted ZnO nanoparticles dissolve in the intracellular environment of triple negative breast cancer cells and cause apoptosis with intratumor heterogeneity. Adv Healthc Mater. 2016;5:1310–1325. doi: 10.1002/adhm.201501012. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y., He L., Yu B., Chen Y., Shen Y. ZnO quantum dots modified by pH-activated charge-reversal polymer for tumor targeted drug delivery. Polymers (Basel) 2018;10:1272. doi: 10.3390/polym10111272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jia Y.P., Shi K., Yang F., Liao J.F., Han R.X. Multifunctional nanoparticle loaded injectable thermoresponsive hydrogel as NIR controlled release platform for local photothermal immunotherapy to prevent breast cancer postoperative recurrence and metastases. Adv Funct Mater. 2020;30:2001095. [Google Scholar]
- 124.Shih Y.J., Chang Y. Tunable blood compatibility of polysulfobetaine from controllable molecular-weight dependence of zwitterionic nonfouling nature in aqueous solution. Langmuir. 2010;26:17286–17294. doi: 10.1021/la103186y. [DOI] [PubMed] [Google Scholar]
- 125.Chen C.Y., Wang H.L. Dual thermo- and pH-responsive zwitterionic sulfobataine copolymers for oral delivery system. Macromol Rapid Commun. 2014;35:1534–1540. doi: 10.1002/marc.201400161. [DOI] [PubMed] [Google Scholar]
- 126.Farkhondeh T., Forouzanfar F., Roshanravan B., Samarghandian S. Curcumin effect on non-amyloidogenic pathway for preventing Alzheimer’s disease. Biointer Res Appl Chem. 2019;9:4085–4089. [Google Scholar]
- 127.Segregur D., Flanagan T., Mann J., Moir A., Karlsson E.M. Impact of acid-reducing agents on gastrointestinal physiology and design of biorelevant dissolution tests to reflect these changes. J Pharm Sci. 2019;108:3461–3477. doi: 10.1016/j.xphs.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 128.Ding K., Li R., Ma Y., Li N., Zhang T. Folate ligand orientation optimized during cell membrane mimetic micelle formation for enhanced tumor cell targeting. Langmuir. 2019;35:1257–1265. doi: 10.1021/acs.langmuir.8b00744. [DOI] [PubMed] [Google Scholar]
- 129.Zhong Y., Meng F., Deng C., Zhong Z. Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy. Biomacromolecules. 2014;15:1955–1969. doi: 10.1021/bm5003009. [DOI] [PubMed] [Google Scholar]
- 130.Dosio F., Arpicco S., Stella B., Fattal E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv Drug Deliv Rev. 2016;97:204–236. doi: 10.1016/j.addr.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 131.Hu X., Guan X., Li J., Pei Q., Liu M. Hybrid polymer micelles capable of cRGD targeting and pH-triggered surface charge conversion for tumor selective accumulation and promoted uptake. Chem Commun (Camb) 2014;50:9188–9191. doi: 10.1039/c4cc04056b. [DOI] [PubMed] [Google Scholar]
- 132.Guan X.G., Hu X.L., Li Z.H., Zhang H., Xie Z.G. cRGD targeted and charge conversion-controlled release micelles for doxorubicin delivery. RSC Adv. 2015;5:22957–22964. [Google Scholar]
- 133.Jinrong Peng Q.Y., Xiao Y. Tumor microenvironment responsive drug-dye-peptide nanoassembly for enhanced tumor-targeting, penetration, and photo-chemo-immunotherapy. Adv Funct Mater. 2019;29 1900004.1–1900004.16. [Google Scholar]
- 134.Noh T., Kook Y.H., Park C., Youn H., Kim H. Block copolymer micelles conjugated with anti-EGFR antibody for targeted delivery of anticancer drug. J Polym Sci Part Pol Chem. 2008;46:7321–7331. [Google Scholar]
- 135.Reddy J.A., Low P.S. Folate-mediated targeting of therapeutic and imaging agents to cancers. Crit Rev Ther Drug Carrier Syst. 1998;15:587–627. [PubMed] [Google Scholar]
- 136.Licciardi M., Craparo E.F., Giammona G., Armes S.P., Tang Y. In vitro biological evaluation of folate-functionalized block copolymer micelles for selective anti-cancer drug delivery. Macromol Biosci. 2008;8:615–626. doi: 10.1002/mabi.200800009. [DOI] [PubMed] [Google Scholar]
- 137.Licciardi M., Giammona G., Du J.Z., Armes S.P., Tang Y.Q. New folate-functionalized biocompatible block copolymer micelles as potential anti-cancer drug delivery systems. Polymer. 2006;47:2946–2955. [Google Scholar]
- 138.Xiong J.A., Meng F.H., Wang C., Cheng R., Liu Z.A. Folate-conjugated crosslinked biodegradable micelles for receptor-mediated delivery of paclitaxel. J Mater Chem. 2011;21:5786–5794. [Google Scholar]
- 139.Yu J.M., Xie X., Xu X.Y., Zhang L., Zhou X.Y. Development of dual ligand-targeted polymeric micelles as drug carriers for cancer therapy in vitro and in vivo. J Mater Chem B. 2014;2:2114–2126. doi: 10.1039/c3tb21539c. [DOI] [PubMed] [Google Scholar]
- 140.Lu Q., Yi M., Zhang M., Shi Z., Zhang S. Folate-conjugated cell membrane mimetic polymer micelles for tumor-cell-targeted delivery of doxorubicin. Langmuir. 2019;35:504–512. doi: 10.1021/acs.langmuir.8b03693. [DOI] [PubMed] [Google Scholar]
- 141.Jiang H.T., Ding K., Meng F.N., Bao L.L., Chai Y.D. Anti-phagocytosis and tumor cell targeting micelles prepared from multifunctional cell membrane mimetic polymers. J Mater Chem B. 2016;4:5464–5474. doi: 10.1039/c6tb00953k. [DOI] [PubMed] [Google Scholar]
- 142.Colombo M., Bianchi A. Click chemistry for the synthesis of RGD-containing integrin ligands. Molecules. 2010;15:178–197. doi: 10.3390/molecules15010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sugahara K.N., Teesalu T., Karmali P.P., Kotamraju V.R., Agemy L. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328:1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gajbhiye K.R., Gajbhiye V., Siddiqui I.A., Gajbhiye J.M. cRGD functionalised nanocarriers for targeted delivery of bioactives. J Drug Target. 2019;27:111–124. doi: 10.1080/1061186X.2018.1473409. [DOI] [PubMed] [Google Scholar]
- 145.Huang P., Liu J., Wang W., Zhang Y., Zhao F. Zwitterionic nanoparticles constructed from bioreducible RAFT-ROP double head agent for shell shedding triggered intracellular drug delivery. Acta Biomater. 2016;40:263–272. doi: 10.1016/j.actbio.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 146.Lin W., Ma G., Yuan Z., Qian H., Xu L. Development of zwitterionic polypeptide nanoformulation with high doxorubicin loading content for targeted drug delivery. Langmuir. 2019;35:1273–1283. doi: 10.1021/acs.langmuir.8b00851. [DOI] [PubMed] [Google Scholar]
- 147.White A.D., Nowinski A.K., Huang W.J., Keefe A.J., Sun F. Decoding nonspecific interactions from nature. Chem Sci. 2012;3:3488–3494. [Google Scholar]
- 148.Liu E.J., Sinclair A., Keefe A.J., Nannenga B.L., Coyle B.L. EKylation: addition of an alternating-charge peptide stabilizes proteins. Biomacromolecules. 2015;16:3357–3361. doi: 10.1021/acs.biomac.5b01031. [DOI] [PubMed] [Google Scholar]
- 149.Yamashita K., Mimori K., Inoue H., Mori M., Sidransky D. A tumor-suppressive role for trypsin in human cancer progression. Cancer Res. 2003;63:6575–6578. [PubMed] [Google Scholar]
- 150.Soreide K., Janssen E.A., Korner H., Baak J.P.A. Trypsin in colorectal cancer: molecular biological mechanisms of proliferation, invasion, and metastasis. J Pathol. 2006;209:147–156. doi: 10.1002/path.1999. [DOI] [PubMed] [Google Scholar]
- 151.Kejin Zhou Y.W., Huang X., Luby-Phelps K., Sumer B.D., Gao J. Ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew Chem Int Ed. 2011;50:6109–6114. doi: 10.1002/anie.201100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang P., Liu J., Wang W., Li C., Zhou J. Zwitterionic nanoparticles constructed with well-defined reduction-responsive shell and pH-sensitive core for “spatiotemporally pinpointed” drug delivery. ACS Appl Mater Interfaces. 2014;6:14631–14643. doi: 10.1021/am503974y. [DOI] [PubMed] [Google Scholar]
- 153.Underhill C. CD44: the hyaluronan receptor. J Cell Sci. 1992;103:293–298. doi: 10.1242/jcs.103.2.293. [DOI] [PubMed] [Google Scholar]
- 154.Swierczewska M., Han H.S., Kim K., Park J.H., Lee S. Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv Drug Deliv Rev. 2016;99:70–84. doi: 10.1016/j.addr.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gao Q.Q., Zhang C.M., Zhang E.X., Chen H.Y., Zhen Y.H. Zwitterionic pH-responsive hyaluronic acid polymer micelles for delivery of doxorubicin. Colloids Surf B Biointerfaces. 2019;178:412–420. doi: 10.1016/j.colsurfb.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 156.Huang Y., Tang Z., Zhang X., Yu H., Sun H. pH-Triggered charge-reversal polypeptide nanoparticles for cisplatin delivery: preparation and in vitro evaluation. Biomacromolecules. 2013;14:2023–2032. doi: 10.1021/bm400358z. [DOI] [PubMed] [Google Scholar]
- 157.Wickens J.M., Alsaab H.O., Kesharwani P., Bhise K., Amin M. Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov Today. 2017;22:665–680. doi: 10.1016/j.drudis.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li Q., Qin Z., Wang Q., Xu T., Yang Y. Applications of genome editing technology in animal disease modeling and gene therapy. Comput Struct Biotechnol J. 2019;17:689–698. doi: 10.1016/j.csbj.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li T., Zhu L., Xiao B., Gong Z., Liao Q. CRISPR-Cpf1-mediated genome editing and gene regulation in human cells. Biotechnol Adv. 2019;37:21–27. doi: 10.1016/j.biotechadv.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 160.Zhou L.Y., He Z.Y., Xu T., Wei Y.Q. Current advances in small activating RNAs for gene therapy: principles, applications and challenges. Curr Gene Ther. 2018;18:134–142. doi: 10.2174/1566523218666180619155018. [DOI] [PubMed] [Google Scholar]
- 161.Zhou L.Y., Qin Z., Zhu Y.H., He Z.Y., Xu T. Current RNA-based therapeutics in clinical trials. Curr Gene Ther. 2019;19:172–196. doi: 10.2174/1566523219666190719100526. [DOI] [PubMed] [Google Scholar]
- 162.Ma C.C., Wang Z.L., Xu T., He Z.Y., Wei Y.Q. The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol Adv. 2020;40:107502. doi: 10.1016/j.biotechadv.2019.107502. [DOI] [PubMed] [Google Scholar]
- 163.He Z.Y., Zhang Y.G., Yang Y.H., Ma C.C., Wang P. In vivo ovarian cancer gene therapy using CRISPR-Cas9. Hum Gene Ther. 2018;29:223–233. doi: 10.1089/hum.2017.209. [DOI] [PubMed] [Google Scholar]
- 164.Tie Y., Zheng H., He Z., Yang J., Shao B. Targeting folate receptor beta positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct Target Ther. 2020;5:6. doi: 10.1038/s41392-020-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xiu K.M., Zhao N.N., Yang W.T., Xu F.J. Versatile functionalization of gene vectors via different types of zwitterionic betaine species for serum-tolerant transfection. Acta Biomater. 2013;9:7439–7448. doi: 10.1016/j.actbio.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 166.Han H.B., Yang J.B., Chen W.Q., Li Q., Yang Y. A comprehensive review on histone-mediated transfection for gene therapy. Biotechnol Adv. 2019;37:132–144. doi: 10.1016/j.biotechadv.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 167.He Z.Y., Men K., Qin Z., Yang Y., Xu T. Non-viral and viral delivery systems for CRISPR-Cas9 technology in the biomedical field. Sci China Life Sci. 2017;60:458–467. doi: 10.1007/s11427-017-9033-0. [DOI] [PubMed] [Google Scholar]
- 168.Wang K., Kievit F.M., Zhang M. Nanoparticles for cancer gene therapy: recent advances, challenges, and strategies. Pharmacol Res. 2016;114:56–66. doi: 10.1016/j.phrs.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 169.Yin H., Kauffman K.J., Anderson D.G. Delivery technologies for genome editing. Nat Rev Drug Discov. 2017;16:387–399. doi: 10.1038/nrd.2016.280. [DOI] [PubMed] [Google Scholar]
- 170.Sun J., Zeng F., Jian H., Wu S. Conjugation with betaine: a facile and effective approach to significant improvement of gene delivery properties of PEI. Biomacromolecules. 2013;14:728–736. doi: 10.1021/bm301826m. [DOI] [PubMed] [Google Scholar]
- 171.Dubruel P., Schacht E. Vinyl polymers as non-viral gene delivery carriers: current status and prospects. Macromol Biosci. 2006;6:789–810. doi: 10.1002/mabi.200600110. [DOI] [PubMed] [Google Scholar]
- 172.Li L., Song L., Liu X., Yang X., Li X. Artificial virus delivers CRISPR-Cas9 system for genome editing of cells in mice. ACS Nano. 2017;11:95–111. doi: 10.1021/acsnano.6b04261. [DOI] [PubMed] [Google Scholar]
- 173.Needham C.J., Williams A.K., Chew S.A., Kasper F.K., Mikos A.G. Engineering a polymeric gene delivery vector based on poly(ethylenimine) and hyaluronic acid. Biomacromolecules. 2012;13:1429–1437. doi: 10.1021/bm300145q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Saraf A., Hacker M.C., Sitharaman B., Grande-Allen K.J., Barry M.A. Synthesis and conformational evaluation of a novel gene delivery vector for human mesenchymal stem cells. Biomacromolecules. 2008;9:818–827. doi: 10.1021/bm701146f. [DOI] [PubMed] [Google Scholar]
- 175.Shujiro Sakaki M.T., Iwasaki Y., Ishihara K. A water-soluble phospholipid polymer as a new biocompatible synthetic DNA carrier. Bull Chem Soc Jpn. 2004;77:2283–2288. [Google Scholar]
- 176.Kitagawa T., Iwase R., Ishihara K., Yamaoka T., Murakami A. Facilitated disassembly of polyplexes composed of self-assembling amphiphilic polycations enhances the gene transfer efficacy. Chem Lett. 2005;34:1478–1479. [Google Scholar]
- 177.Chiba N., Ueda M., Shimada T., Jinno H., Watanabe J. Development of gene vectors for pinpoint targeting to human hepatocytes by cationically modified polymer complexes. Eur Surg Res. 2007;39:23–34. doi: 10.1159/000098437. [DOI] [PubMed] [Google Scholar]
- 178.Ahmed M., Bhuchar N., Ishihara K., Narain R. Well-controlled cationic water-soluble phospholipid polymer-DNA nanocomplexes for gene delivery. Bioconjug Chem. 2011;22:1228–1238. doi: 10.1021/bc2001159. [DOI] [PubMed] [Google Scholar]
- 179.Lam J.K., Ma Y., Armes S.P., Lewis A.L., Baldwin T. Phosphorylcholine-polycation diblock copolymers as synthetic vectors for gene delivery. J Control Release. 2004;100:293–312. doi: 10.1016/j.jconrel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 180.Licciardi M., Tang Y., Billingham N.C., Armes S.P., Lewis A.L. Synthesis of novel folic acid-functionalized biocompatible block copolymers by atom transfer radical polymerization for gene delivery and encapsulation of hydrophobic drugs. Biomacromolecules. 2005;6:1085–1096. doi: 10.1021/bm049271i. [DOI] [PubMed] [Google Scholar]
- 181.Lam J.K., Armes S.P., Lewis A.L., Stolnik S. Folate conjugated phosphorylcholine-based polycations for specific targeting in nucleic acids delivery. J Drug Target. 2009;17:512–523. doi: 10.1080/10611860903023312. [DOI] [PubMed] [Google Scholar]
- 182.Ahmed M., Narain R. The effect of polymer architecture, composition, and molecular weight on the properties of glycopolymer-based non-viral gene delivery systems. Biomaterials. 2011;32:5279–5290. doi: 10.1016/j.biomaterials.2011.03.082. [DOI] [PubMed] [Google Scholar]
- 183.Ahmed M., Jawanda M., Ishihara K., Narain R. Impact of the nature, size and chain topologies of carbohydrate-phosphorylcholine polymeric gene delivery systems. Biomaterials. 2012;33:7858–7870. doi: 10.1016/j.biomaterials.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 184.Dai F., Liu W. Enhanced gene transfection and serum stability of polyplexes by PDMAEMA-polysulfobetaine diblock copolymers. Biomaterials. 2011;32:628–638. doi: 10.1016/j.biomaterials.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 185.Wang W., Nan W.J., Sun L., Liu W.G. A systemic gene vector constructed by zwitterionic polymer modified low molecular weight PEI. React Funct Polym. 2013;73:993–1000. [Google Scholar]
- 186.Yang J., Zhang P., Tang L., Sun P., Liu W. Temperature-tuned DNA condensation and gene transfection by PEI-g-(PMEO(2)MA-b-PHEMA) copolymer-based nonviral vectors. Biomaterials. 2010;31:144–155. doi: 10.1016/j.biomaterials.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 187.Shih Y., Venault A., Tayo L.L., Chen S.H., Higuchi A. A zwitterionic-shielded carrier with pH-modulated reversible self-assembly for gene transfection. Langmuir. 2017;33:1914–1926. doi: 10.1021/acs.langmuir.6b03685. [DOI] [PubMed] [Google Scholar]
- 188.Chen J., Dong X., Feng T., Lin L., Guo Z. Charge-conversional zwitterionic copolymer as pH-sensitive shielding system for effective tumor treatment. Acta Biomater. 2015;26:45–53. doi: 10.1016/j.actbio.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 189.Fu C., Lin L., Shi H., Zheng D., Wang W. Hydrophobic poly (amino acid) modified PEI mediated delivery of rev-casp-3 for cancer therapy. Biomaterials. 2012;33:4589–4596. doi: 10.1016/j.biomaterials.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 190.Miller J.B., Zhang S., Kos P., Xiong H., Zhou K. Non-viral CRISPR/cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew Chem Int Ed Engl. 2017;56:1059–1063. doi: 10.1002/anie.201610209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li Y., Cheng Q., Jiang Q., Huang Y., Liu H. Enhanced endosomal/lysosomal escape by distearoyl phosphoethanolamine-polycarboxybetaine lipid for systemic delivery of siRNA. J Control Release. 2014;176:104–114. doi: 10.1016/j.jconrel.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 192.Li Y., Liu R., Shi Y., Zhang Z., Zhang X. Zwitterionic poly(carboxybetaine)-based cationic liposomes for effective delivery of small interfering RNA therapeutics without accelerated blood clearance phenomenon. Theranostics. 2015;5:583–596. doi: 10.7150/thno.11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Han X., Lu Y., Xie J., Zhang E., Zhu H. Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions. Nat Nanotechnol. 2020;15:605–614. doi: 10.1038/s41565-020-0693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang L., Wang Z., Ma G., Lin W., Chen S. Reducing the cytotoxity of poly(amidoamine) dendrimers by modification of a single layer of carboxybetaine. Langmuir. 2013;29:8914–8921. doi: 10.1021/la400623s. [DOI] [PubMed] [Google Scholar]
- 195.Kong L., Wu Y., Alves C.S., Shi X. Efficient delivery of therapeutic siRNA into glioblastoma cells using multifunctional dendrimer-entrapped gold nanoparticles. Nanomedicine (Lond) 2016;11:3103–3115. doi: 10.2217/nnm-2016-0240. [DOI] [PubMed] [Google Scholar]
- 196.Xiong Z., Alves C.S., Wang J., Li A., Liu J. Zwitterion-functionalized dendrimer-entrapped gold nanoparticles for serum-enhanced gene delivery to inhibit cancer cell metastasis. Acta Biomater. 2019;99:320–329. doi: 10.1016/j.actbio.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 197.Dai F.Y., Wang P.F., Wang Y., Tang Lei, Yang J.H. Double thermoresponsive polybetaine-based ABA triblock copolymers with capability to condense DNA. Polymer. 2008;49:5322–5328. [Google Scholar]
- 198.Carr L.R., Jiang S. Mediating high levels of gene transfer without cytotoxicity via hydrolytic cationic ester polymers. Biomaterials. 2010;31:4186–4193. doi: 10.1016/j.biomaterials.2010.01.110. [DOI] [PubMed] [Google Scholar]
- 199.Sinclair A., Bai T., Carr L.R., Ella-Menye J.R., Zhang L. Engineering buffering and hydrolytic or photolabile charge shifting in a polycarboxybetaine ester gene delivery platform. Biomacromolecules. 2013;14:1587–1593. doi: 10.1021/bm400221r. [DOI] [PubMed] [Google Scholar]
- 200.Zhang J., Wang Z., Lin W., Chen S. Gene transfection in complex media using PCBMAEE-PCBMA copolymer with both hydrolytic and zwitterionic blocks. Biomaterials. 2014;35:7909–7918. doi: 10.1016/j.biomaterials.2014.05.066. [DOI] [PubMed] [Google Scholar]