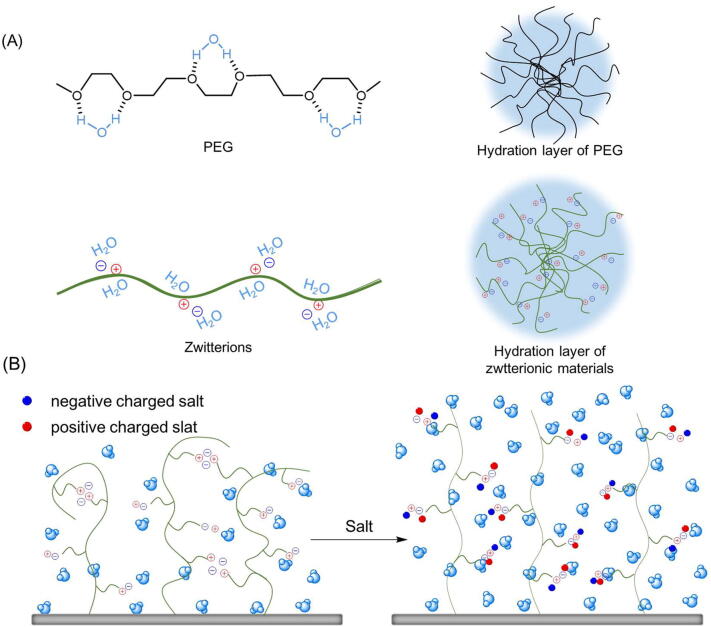
Fig. 1.
Schematic diagram of hydration shell formed by PEG and zwitterions separately. (A) PEG interact with H2O molecules via hydrogen bonding, while zwitterions attract H2O molecules through the powerful ion–dipole interaction, so that to form stronger hydration layers to prevent bio-adherent. (B) Incorporation of salt disrupts the previous electrostatic attraction of the charge pairs of intra-monomer, intra-chain and inter-chain, making the conformation of zwitterionic brushes from shrinking to stretching relatively.