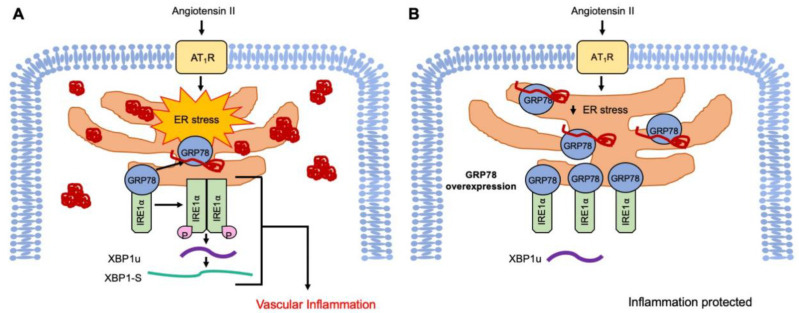
Figure 5.
Proposed mechanisms for GRP78 mitigation of AngII-induced dysfunctional proteostasis and subsequent vascular inflammation. (A) Angiotensin II (AngII) via angiotensin II type 1 receptor (AT1R) induces ER stress. The proteostasis mechanism appears insufficient leading to protein aggregation in VSMC. GRP78 detaches from IRE1α to bind nascent misfolded peptide chains, and in turn leads to IRE1α dimerization and autophosphorylation. This leads to XBP1-S alternative splicing and potential UPRosome initiation, which activates VSMC proinflammatory phenotype, contributes to immune cell infiltration in the vasculature, and the overall progression of CVD. (B) The overexpression of GRP78 mitigates peptide misfolding into aggregates under AngII stimulation, while also curbing UPR signaling by maintaining its attachment to IRE1α and protecting monocyte adhesion/vascular inflammation.