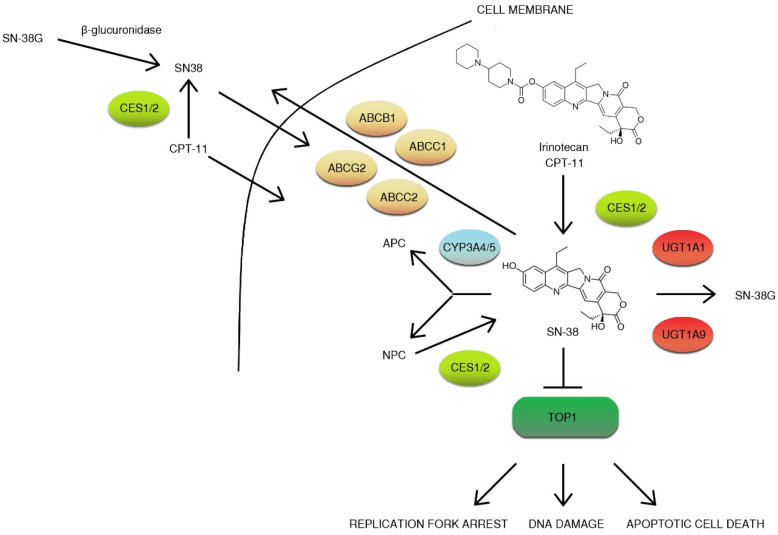
Figure 5.
Overview of irinotecan metabolism. CPT-11 is a prodrug that is converted to active metabolite ethyl-10-hydroxy-camptothecin (SN-38) by liver carboxylesterase converting enzymes (CES1/2) and is then transported to the liver by 1B1 polypeptide (OATP1B1) and inactivated by microsomal uridine 5′-diphospho-glucuronosyltransferase enzymes (UGTs): UGT1A1 and UGT1A9. Irinotecan is transported to bile by a group of the ATP-binding cassette transporters (ABC transporters): ABCB1, ABCC1, ABCC2, and ABCG2. Irinotecan is efficiently metabolized by cytochrome P450 enzymes: CYP3A4 and CYP3A5. This results in the generation of less active metabolites APC (7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino] carbonyloxycamptothecin) and NPC (7-ethyl-10-[4-amino-1-piperidino] carbonyloxycamptothecin). NPC (but not APC) can be further converted to SN-38 by CES1, and CES2 gut microbiota may also participate in irinotecan metabolism by the production of β-glucuronidase, which catalyzes the breakdown of SN-38G to SN-38. The inhibition of topoisomerase I (TOP1) results in DNA damage, replication fork arrest, and apoptotic cell death [53,54,55,56,57,58,59,60,61,62].