Abstract
Two alternative methods for the production of compost from certain category 3 animal by‐products (catering waste and processed foodstuffs of animal origin) were assessed. The first proposed a minimum temperature of 55°C for 72 h; the second 60°C for 48 h, each with a maximum particle size of 200 mm. The proposed composting processes were assessed by the BIOHAZ Panel for their efficacy to achieve a reduction of 5 log10 of Enterococcus faecalis or Salmonella Senftenberg (775W, H2S negative) and a 3 log10 reduction of the infectivity titre of thermoresistant viruses, such as parvovirus, in the composted material, as set out in Annex V, Chapter 3, Section 2 of Commission Regulation (EU) No 142/2011. The assessment of the BIOHAZ Panel exclusively focused on the ABP raw materials (catering waste and processed foodstuffs) intended for human consumption. The applicant did not provide any validation experiments with direct measurement of the reduction of viability of endogenous indicators or spiked surrogate bacteria. However, from thermal inactivation parameters reported in the literature, it can be concluded that the proposed composting standards can achieve at least a 5 log10 reduction of Enterococcus faecalis or Salmonella Senftenberg 775W. The applicant did not consider thermoresistant viruses as a relevant hazard and therefore did not provide any data from direct measurements of the reduction of infectivity of spiked thermoresistant viruses, nor provide data from validation studies undertaken at national level or data from literature supporting the efficacy of the proposed composting standards on thermoresistant viruses. However, thermoresistant viruses should be considered to be a relevant hazard in this context and validation data should have been provided accordingly. The BIOHAZ Panel considers that the evidence provided by the applicant does not demonstrate that the requirements of Annex V, Chapter 3, Section 2 of Commission Regulation (EU) No 142/2011 are achieved.
Keywords: Compost, catering waste, Cat. 3 ABPs, alternative method
Summary
On 5 September 2019, the European Food Safety Authority (EFSA) received from the Belgian Competent Authority (Federal Agency for the Safety of the Food Chain), the application (mandate and technical dossier) (EFSA‐Q‐2019‐00583) under Regulation (EU) No 1069/2009 and Regulation (EU) No 142/2011 referring to the evaluation of an alternative method for the production of compost from category 3 animal by‐products (ABPs) submitted by the European Compost Network (ECN) (hereinafter referred to as the applicant).
The category 3 ABPs in question are defined in Article 10 of Regulation (EU) No 1069/2009 as:
(f) products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arise (which have undergone processing as defined in Article 2(1)(m) of Regulation (EU) No 852/2004).
(p) catering waste (other than catering waste from means of transport operating internationally).
The standard transformation parameters for the composting of category 3 ABPs are detailed in Section 1, Chapter III, Annex V of Regulation (EU) No 142/2011 and shall be carried out according to the following processing standards:
maximum particle size before entering the composting reactor: 12 mm;
minimum temperature in all material in the reactor: 70°C; and
minimum time without interruption: 60 min.
The applicant presented two new alternative methods of compost production:
Standard 1:
maximum particle size of ABP before entering the tunnel: 200 mm;
minimum temperature in all material in the tunnel: 55°C; and
minimum exposure time in the tunnel without interruption: 72 h.
Standard 2:
maximum particle size of ABP before entering the tunnel: 200 mm;
minimum temperature in all material in the tunnel: 60°C; and
minimum exposure time in the tunnel without interruption: 48 h.
The raw material to be treated is biowaste, as described in Directive (EU) 2018/851 amending Directive (EU) 2008/98, meaning biodegradable garden and park waste, food and kitchen waste from households, offices, restaurants, wholesale, canteens, caterers and retail premises and comparable waste from food processing plants. The components of biowaste that are considered to be ABP, as detailed in Regulation (EU) No 1069/2009, are catering waste (except waste from means of transport operating internationally) and processed foodstuffs of animal origin that are no longer fit for human consumption. The assessment of the BIOHAZ Panel does not address biodegradable garden and park waste, which are also included in the definition of biowaste.
In relation to hazard identification, the approach taken by the applicant was to provide a list of biological hazards which may enter the composting process (Toxoplasma, Campylobacter, pathogenic E. coli, Salmonella, Listeria monocytogenes and Clostridium perfringens) and a list of biological hazards that are unlikely to enter the composting process (Scrapie and BSE agents, the viruses causing Food and Mouth Disease, Classical Swine Fever, African Swine Fever, Swine Vesicular Disease and Newcastle Disease, Clostridium botulinum and Trichinella spiralis). The BIOHAZ Panel considered that other pathogenic sporulating and non‐sporulating bacteria (e.g. Clostridioides difficile, Staphylococcus aureus, Enterococcus faecalis, E. faecium) and some viruses, including thermoresistant viruses, such as porcine parvovirus and circovirus and chicken anaemia virus, are important hazards, which may also enter the composting process and should also be taken into account.
The applicant did not perform any validation experiments with direct measurement of the reduction of viability/infectivity of endogenous indicator or spiked surrogate microorganisms and/or viruses, but provided: (i) supporting information from the literature on heat resistance of bacterial biological hazards; (ii) treatment temperature/time equivalence calculations based on establishing a relationship between the heat tolerance of tomato seeds and the heat tolerance of pathogenic bacteria; (iii) the findings of some validation studies carried out at commercial scale composting plants across Europe; and (iv) modelling and experimental data based on the penetration of heat in packaged food and composted wood.
In relation to (i), the BIOHAZ Panel concluded that the proposed treatment standards, if maintained at or above the target temperature during the whole composting process and applied homogeneously in the composting tunnel, would be able to inactivate more than 5 log10 of E. faecalis or S. Senftenberg 775W in the material to be treated, as required in Section 2, Chapter III, Annex V, of Regulation (EU) No 142/2011.
In relation to (ii), the BIOHAZ Panel concluded that an extrapolation from the behaviour of tomato seeds to that of biological hazards is not a valid comparison.
In relation to (iii), the validation studies carried out at commercial scale composting plants in Belgium, Portugal and the United Kingdom showed reductions for E. faecalis exceeding the 5 log10 required in Annex V, Chapter 3, Section 2 of Regulation (EU) No 142/2011. However, in approximately half of the compost plants tested in the Dutch validation studies, supplied as additional information, the 5 log10 reduction of E. faecalis was not achieved, which demonstrates that, in practice, variability in the accomplishment of microbial reduction targets does occur. Moreover, in some of the validation studies provided, the process parameters (temperature, time or particle size) were different from the alternative transformation parameters of the processes under assessment and none of the validation studies submitted with the application evaluated the level of reduction of thermoresistant viruses, such as parvovirus.
In relation to (iv), the heat penetration experiments in composted wood show that for particle sizes of 200 mm, it can take around 24 h to reach the composting temperature. The results of heat penetration modelling, using methodology described for estimating heat penetration and pasteurisation times of packaged foods, show that it takes between approximately 18 h and 33.36 h for a particle of 200 mm to reach 60°C. It is uncertain how applicable these models are to the composting processes, especially in an industrial context.
The BIOHAZ Panel considers that the generic hazard analysis critical control point (HACCP) plan provided and the information about the risks of the interdependent processes and those associated with the intended end use are generally appropriate and can be the basis for the validation and verification of the process once implemented at industrial level.
The applicant provided a description of the risks associated with leachate from the process and storage of raw materials, as well as the procedures that would be implemented for dealing with these risks. Transport of the end product should be considered.
The end product of the process is compost, which according to the applicant may be used as a fertiliser and/or soil improver (it will be used on agricultural land, for landscaping projects and for horticultural uses). The applicant envisages the establishment of the end point of the process at the composting plant when the end product complies with microbial testing standards. Provided that the alternative method is capable of achieving a risk reduction level equivalent to that of the method in the Regulation and that these microbial standards are met, no additional risks associated with the intended end use of the product are foreseen.
In conclusion, the BIOHAZ Panel considers that the evidence provided by the applicant does not demonstrate that the requirements of Annex V, Chapter 3, Section 2 of Commission Regulation (EU) No 142/2011 are achieved.
1. Introduction
1.1. Background and Terms of Reference as provided by the Requestor
On the 5th of September 2019, the European Food Safety Authority (EFSA) received from the Belgian Competent Authority (Federal Agency for the Safety of the Food Chain) the application (mandate and technical dossier) (EFSA‐Q‐2019‐00583) under Regulation (EU) No 1069/2009 and Regulation (EU) No 142/2011 referring to the evaluation of an alternative method for the production of compost from category 3 animal by‐products (ABPs) submitted by the European Compost Network (ECN) (hereinafter referred to as the applicant). The category 3 ABPs in question are defined in Article 10 of Regulation (EU) No 1069/2009 as:
(f) Products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arise, (which have undergone processing as defined in Article 2(1)(m) of Regulation (EU) No 852/2004).
(p) Catering waste (other than catering waste from means of transport operating internationally).
The applicant submitted an application following the procedure for authorisation of an alternative method of use or disposal of animal by‐products or derived products, laid down in Article 20 of the Regulation (EU) No 1069/2009.
During the completeness check, performed according to Regulation (EU) No 1069/2009, it was noticed that some information was missing or incomplete. Therefore, the dossier could not be considered complete. On the 17th of October 2019, EFSA sent a letter to the applicant with 7 requests:
In relation to the full description of the process the applicant was asked to provide information on the microbiological criteria to be used for the by‐products or a scientific justification for its omission.
In relation to the full description of the material to be treated, the applicant was asked to clarify the content of Section 3.1.2.1 (b) (of the application) or delete the reference if not appropriate.
In relation to the level of risk reduction, the applicant was asked to provide the missing information or a scientific justification for the omission in the application of results accompanied by evidence including (i) the methodology used; (ii) the number of samples analysed and their representativeness; (iii) the justification for the number of tests performed and the selection of measuring points; (iv) the repeatability and statistical variability of the detection methods applied.
In relation to the HACCP Plan, the applicant was asked to complete and review the HACCP plan, as appropriate.
The applicant was asked to supply all the cited references.
The applicant was asked to ensure that all documents were correctly named.
The applicant was asked to confirm that no claims for confidentiality are made for the application.
On the 25th of November 2019, EFSA received the missing information concerning the application EFSA‐Q‐2019‐00583, following its request dated the 17th of October 2019. After checking the content of the full dossier, EFSA considered that the application EFSA‐Q‐2019‐00583 was valid on the 12th of December 2019. According to Regulation (EU) No 1069/2009, EFSA was to respect the deadline of 6 months to deliver a scientific opinion. Therefore, the scientific opinion was to be delivered by the 12th of June 2020.
On the 15th of April 2020 EFSA requested the following additional information from the applicant, while suspending the scientific process:
The Working Group (WG) noted that although certain viruses (Foot and Mouth Disease, Classical Swine Fever, African Swine Fever, Swine Vesicular Disease & Newcastle Disease) were mentioned in Section 4.1.2.1 Hazard Identification (of the application), they were considered unlikely to enter the composting process. In addition, the risk reduction criteria (reductions in viral levels) for these and potentially other thermoresistant viruses achieved as a result of the proposed composting parameters have not been included in Section 4.1.2.1 D Level of Risk Reduction. Please provide evidence of the level of risk reduction of thermoresistant viruses as a result of the proposed composting parameters (Standard 1) 55°C for 72 h with a maximum particle size of 200 mm and (Standard 2) 60°C for 48 h with a maximum particle size of 200 mm.
In Table 1 the applicant has presented data from 9 Dutch composting plants. However, in the body of the text, the applicant refers to a national study commissioned by the Dutch Waste Management Association which describes risk reduction trials in 21 plants where the 21 plants demonstrated a 4.7 log units reduction for Enterococcus (7.1 down to 2.4). Fifteen of the 21 plants showed a reduction of almost 5 log units or more and met the ABP requirements. Please provide a copy of the full report on the study carried out by the Dutch Waste Management Association (2006).
In the appendices to the application there are a number of reports that were part of the national authorisation processes for composting plants in Portugal and Belgium but not the UK and the Netherlands. The Dutch report has been requested in point 2 above. Please provide the report from the authorisation of the Envar composting plant in the UK (as referred to in Table 1).
Table 1.
Summary of Validation of Compost Plants According to ABP Regulation (EU) No 142 of 2011
| Description of Composting System and Tunnel ID | Temperature (°C) | Time (Hours) | Particle Size limit (mm) | Log Reduction for Enterococcus faecalis | Log Reduction for Salmonella Senftenberg |
|---|---|---|---|---|---|
| Lipor Tunnel (No 15) pre‐composting stage | 63.5 | 48 | 150 | > 7.46 | |
|
Lipor Tunnel (No 8) pre‐composting stage |
61 | 48 | 150 | > 7.60 | |
| Lipor Tunnel (No 12) pre‐composting stage | 60 | 48 | 150 | > 7.66 | |
|
Lipor Tunnel (No 3) post composting stage |
60 | 24 | 60 | > 7.15 | |
|
Lipor Tunnel (No 3) post composting stage |
60 | 48 | 60 | > 7.15 | |
|
Lipor Tunnel (No 3) post composting stage |
60 | 36 | 60 | > 7.90 | |
| Plant A, Belgium | 55 | 48 | none | 7 | |
| Plant B, Belgium | 55 | 48 | none | 7 | |
| Plant C, Belgium | 60 | 24 | < 120 | 7 | |
| Attero Deurne, NL | 60 | 24 | none | 5.65 | |
| Attero Maastricht, NL | 60 | 24 | none | 5.5 | |
| Attero Venlo, NL | 60 | 72 | none | 7.3 | |
| ARN, NL | 57.5 | 24 | 60 | 7.18 | |
| Valor, St. Oedenrode, NL | 56 | 24 | 250 | 6.51 | |
| Valor, Bladel, NL | 59 | 24 | 250 | 6.54 | |
| Twence, NL | 51.2 | 24 | 60 | 6.38 | |
| Meerlanden, NL | 58 | 24 | 60 | 6.04 | |
| van Vliet, NL | 58 | 20 | None | 7.18 | |
| Envar, UK | 60 | 48 | 400 | 6 | |
| Envar, UK | 60 | 24 | 400 | > 7 |
The applicant made a submission in response to the request made by EFSA for additional information on the 13th of May 2020. The scientific assessment therefore restarted on that day with the new deadline for the delivery of the opinion being the 10th of July 2020.
The standard transformation parameters for the composting of Cat 3 ABPs are detailed in Section 1, Chapter III, Annex V of Regulation (EU) No 142/2011. The composting of Cat 3 ABPs shall be carried out according to the following processing standards:
maximum particle size before entering the composting reactor: 12 mm;
minimum temperature in all material in the reactor: 70°C; and
minimum time without interruption: 60 min.
This application presents two new methods of compost production. Raw materials include products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arise, which have undergone processing as defined in Article 2(1)(m) of Regulation (EU) No 852/2004, and catering waste (other than catering waste from means of transport operating internationally). Additional feedstocks intended for use and that are not subject to Regulation (EU) No 1069/2009 and Regulation (EU) No 142/2011 include garden waste and other organic bulking materials. The proposed new methods for the composting of these Cat. 3 ABPs consist of the following parameters:
Standard 1:
maximum particle size of ABP before entering the tunnel: 200 mm;
minimum temperature in all material in the tunnel: 55°C; and
minimum exposure time in the tunnel without interruption: 72 h.
Standard 2:
maximum particle size of ABP before entering the tunnel: 200 mm;
minimum temperature in all material in the tunnel: 60°C; and
minimum exposure time in the tunnel without interruption: 48 h.
As set out in Article 20 of Regulation (EU) No 1069/2009, EFSA is required to assess whether the methods submitted ensure that any risks to public or animal health are reduced to a degree that is at least equivalent to that achieved by the processing methods that have already been approved for the same category of ABP.
2. Data and methodologies
2.1. Data
The data used in the assessment were provided by the Applicant as requested in Annex VII of Commission Regulation (EU) No 142/2011 and its amendment by Commission Regulation (EU) No 749/2011. A process flow diagram, with a description of the proposed alternative process, and a Hazard Analysis and Critical Control Point (HACCP) plan were included in the application dossier as well as a description of validation exercises conducted in commercial scale composting plants across Europe, where validation was carried out in accordance with the procedure provided for in Annex V, Chapter 3, Section 2 of Regulation (EU) No 142/2011. Additional data were also submitted by the applicant in response to a request for additional information as described above. The report submitted by the Competent Authority (CA) related to the application was also considered. Relevant scientific papers provided by experts of the Working Group (WG) were also considered during the assessment.
2.2. Methodologies
The EFSA Panel on Biological Hazards (BIOHAZ) evaluated the application for an alternative method for production of compost from products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arise, which have undergone processing as defined in Article 2(1)(m) of Regulation (EC) No 852/2004, and catering waste (other than catering waste from means of transport operating internationally).
The steps set out in the ‘EFSA Scientific Opinion on the format for applications for new alternative methods for ABP’ (EFSA BIOHAZ Panel, 2010) were followed in the assessment. These steps are:
full description of the process
full description of the material to be treated
hazard identification
level of risk reduction
HACCP plan
risk associated with interdependent processes
risk associated with the intended end use of the product.
As set out in Article 20 of European Union Regulation (EU) No 1069/2009, EFSA is required to assess whether the method submitted ensures that the risks to public or animal health are:
‘controlled in a manner which prevents their proliferation before disposal in accordance with this Regulation or the implementing measures thereof’
or ‘reduced to a degree which is at least equivalent, for the relevant categories of animal by‐products, to the processing methods laid down pursuant to point (b) of the first subparagraph of Article 15(1)’.
This requirement for applications is described in the EU Regulation (EU) No 142/2011 implementing Regulation (EU) No 1069/2009 and amended by Commission Regulation (EU) No 749/2011. According to point 2(d), Chapter II, Annex VII of Regulation 142/2011, any application for the evaluation of alternative methods shall ‘show that the most resistant biological hazards associated with the category of materials to be processed are reduced in any products generated during the process, including the wastewater, at least to the degree achieved by the processing standards laid down in this Regulation for the same category of animal by‐products. The degree of risk reduction must be determined with validated direct measurements, unless modelling or comparisons with other processes are acceptable’.
The risk reduction achieved as a result of the standard processing methods of Category 1, 2 & 3 ABP materials, as described in the regulation, is not specified. Therefore, no definitive standards have been set down in relation to risk reduction for alternative methods for ABP composting. However, Annex V, Chapter 3, Section 2 of Regulation (EU) No 142/2011 highlights that the competent authority (in a Member State) may authorise the use of parameters other than the standard transformation parameters, provided that the applicant for such use demonstrates that such parameters ensure adequate reduction of biological risks. That demonstration shall include a validation, which shall be carried out in accordance with the following requirements:
Identification and analysis of possible hazards
A risk assessment, which evaluates how the specific transformation conditions referred to in point (a) are achieved in practice under normal and atypical situations
Validation of the intended process by measuring the reduction of viability/infectivity of endogenous or test indicator organisms
-
The validation of the intended process referred to in point (c) must demonstrate that the process achieves the following overall risk reduction:
-
–
a reduction of 5 log 10 of Enterococcus faecalis or Salmonella Senftenberg (775W, H2S negative), and
-
–
a reduction of infectivity titre of thermoresistant viruses such as parvovirus by at least 3 log 10 , whenever they are identified as a relevant hazard.
-
–
Designing a complete control programme including procedures for monitoring the functioning of the process referred to in point (c).
Measures ensuring continuous monitoring and supervision of the relevant process parameters fixed in the control programme when operating the plant.
The BIOHAZ Panel has in the past used the standards mentioned in point (d) above for assessing the risk to human and animal health from dead‐in‐shell chicks (Cat. 2 material) when used as a raw material for the production of compost using the method approved for Cat. 3 material (EFSA, 2015). It was decided by the BIOHAZ Panel, following consultation with the European Commission, to follow the same approach in the evaluation of the current application.
In relation to viruses, the approach to be followed was to assess whether the proposed alternative methods achieved a reduction of infectivity of at least 3 log10 for the most thermoresistant virus which could be present in the material to be treated. The hazards considered for the assessment are exclusively those which may pose a risk to human or animal health and which may be present in the material to be treated.
3. Assessment
3.1. Full Description of the Process
3.1.1. Full Description of the Process (as provided by the applicant)
The description of the process, presented in the current section, has been extracted from the application, edited for clarity and abridged in places for brevity.
The ECN is proposing that the category 3 materials listed in ‘B. Full Description of the Material to be Treated’ of this report are the only ABP feedstock used in a compost plant equipped with a composting tunnel (see Figure 1).
Figure 1.
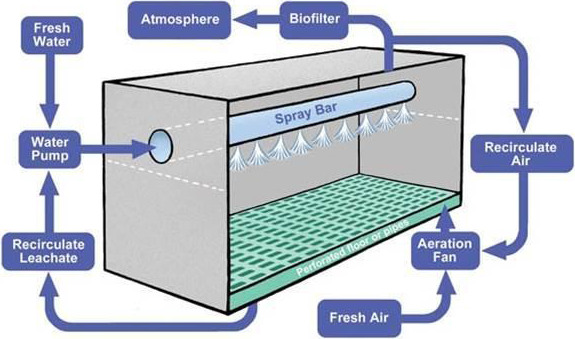
Typical Schematic of a Composting Tunnel (provided by the applicant)
The material must meet the minimum requirements in compliance with the two proposed ECN standards for tunnel composting of catering waste and foods of animal origin; Standard 1: a maximum particle size of ABP 200 mm, a minimum temperature in all material in the tunnel unit of 55°C and a minimum exposure time in the tunnel unit without interruption of 72 h. Standard 2: a maximum particle size of ABP before entering the tunnel of 200 mm, a minimum temperature in all material in the tunnel unit of 60°C and a minimum exposure time in the tunnel unit without interruption of 48 h.
The material flow in the composting process is as follows:
-
1
Intake of Feedstock
Catering waste and products of animal origin will be accepted once it is from an approved feedstock supplier.
-
2
Storage
The feedstock will be stored in a manner which prevents access by vermin.
-
3
Mixing/Blending
The feedstock will be prepared by blending with other non‐ABP feedstocks and chopping into smaller pieces to ensure the ABP material is less than 200 mm in size.
-
4
Composting/Hygienisation
The blended feedstock will be placed in the tunnel for composting and hygienisation. Hygienisation or sanitation of the feedstock occurs as a result of heat generated by microbes during the thermophilic phase of composting. If the moisture needs to be adjusted, liquids from the plant might be used at this stage before hygienisation. Any wastewater/leachate generated from the composting process can only be reused at the start of the composting process before hygienisation. After hygienisation, only clean water can be used.
-
5
Post Sanitisation Treatment & Screening
After the thermophilic or high temperature composting phase, which shall include either the 48 h (temperature > 60°C) or 72 h (temperature > 55°C) standard, the compost is moved with a clean loader to avoid cross‐contamination to further processing or screening. Screening is done to remove impurities. This is done in a separate area from the raw feedstock to prevent cross‐contamination of pathogens. It is important to note that the thermal process conditions providing a temperature range of > 55°C in most composting systems are kept for at least 10 days and, depending on the material mix, humidity and air supply, may last up to several weeks. This contributes to further security with respect to pathogen eradication.
-
6
Dispatch
Once the compost has met all the necessary limits for Escherichia coli, immediately after transformation, and Salmonella, in the final product, it will be dispatched to end users.
The by‐products generated in the process are:
Water vapour and carbon dioxide, which are emitted to the air during composting
Leachate, which is generated from the composting tunnels and from wash water used to clean trucks/floor/machines in the reception hall and is typically used in the composting process prior to hygienisation.
Sanitised rejects (e.g. plastic/glass, screening overs), which are removed at the end of the process
The parameters that are critical for the inactivation of the pathogens in relation to the process are:
Time–Temperature
Temperature and duration are important factors for pathogen inactivation. It is claimed by the applicant that the proposed time–temperature regime of the two ECN standards are sufficient to inactivate pathogens that might possibly be present in the allowed feedstock; this is outlined in Section C (Risk Reduction) of the application. Temperature profiles during composting can be affected by:
Feedstock Preparation
Special attention should be focused on the preprocessing stage. Getting the right mix of feedstock materials is perhaps the most important step in the composting process. It is vital that the composition of the feedstock is adjusted so that optimum conditions for composting are created. Optimum composting conditions will result in more efficient microbial degradation of organic matter and hence more heat generation. In addition, it is essential that feedstocks are blended sufficiently so that a uniform feedstock is created. A uniform feedstock helps to minimize temperature fluctuations and variability within the composting mass.
The addition of green waste/woodchips/oversize material to catering waste serves several functions including:
Improving the structure of the compost pile by providing air spaces within the pile. This facilitates aeration through piles during composting.
Absorbing moisture, especially for wet or high moisture feedstocks. This is important so that wetter feedstock materials can be dried out to a point where they can be composted aerobically. If the material is too wet, the air spaces fill up with water, promoting anaerobic conditions, reducing heat production and promoting the generation of foul odours.
Moisture
If the material is too dry, biological decomposition will be slow or may even stop. If the material is too wet, aerobic composting will be turned into anaerobic conditions and fermentation may be reduced or stopped. In both cases, the temperature will not reach the targeted minimum value.
The ABPs to be processed will be mainly catering waste from households which are typically drier than catering waste from restaurants, which are usually wet and sloppy. Attention to the moisture content of waste from restaurants will be required by operators.
For all feedstock materials, the moisture level should be adjusted prior to composting as the microorganisms need some water to thrive.
Aeration/Particle Size/Porosity
Optimal aeration is provided by a fan in the hygienisation tunnel (see Figure 1). The tunnel composting system is a static system aerated evenly from beneath. Aeration is provided by a fan that extracts the warm air from the roof. If the compost is not sufficiently aerated, the process is slowed and the insufficient air supply leads to anaerobic conditions. The target temperature in the proposed standards will not be reached. If the material has too large a particle size, microorganisms will multiply more slowly and the temperature will not rise fast enough. If the material is too small, air distribution will be reduced in the compost mass, leading to locally anaerobic conditions and lower temperatures.
The particle size affects the time to compost and indirectly aeration. A general rule of thumb is the smaller the particle, the faster it will decay. This has to do with surface area and the ability of microorganisms to access nutrients in the feedstock materials. Conversely, large woody materials decay very slowly and would need to be shredded into smaller pieces to increase the surface area for them to decay efficiently. Furthermore, if the particle size is too small, then there will not be sufficient air space in the piles to promote passive aeration; this can only partly be overcome in tunnel systems with powerful aeration fans.
Porosity is the amount of air space in a blended feedstock mixture or compost pile. Piles with high porosity encourage air flow, while piles with low porosity limit or restrict air flow. So, porosity is crucial to maintain aerobic conditions which in turn reduce the generation of foul odours caused by anaerobic conditions. Structural bulking materials, such as wood chips, are used to create porosity. These larger woody materials typically do not break down as fast as other non‐woody materials and can persist till the end of the composting process. They are typically removed from the finished compost at the end of the process with the use of a screen. These screening overs (rejects) can then be reused in the composting process and introduced into new batches of compost as a structural bulking material and as an inoculant.
The technical data of the equipment used in the relevant process steps are presented in Table A.1 (in the annex).
Table A.1.
Technical Data of the Equipment Used
| Factors | Tunnel Composting |
|---|---|
| Tunnel | The tunnel will be of concrete or other non‐corrosive construction as an enclosed vessel |
| Water content at start‐up in the feedstock mixture | 50–65% |
| Watering | As required during the process. During the post‐hygienisation phase, only clean water can be added |
| Ventilation | Forced aeration is provided by an aeration floor beneath the mass in the tunnel. The warm air is recirculated |
| Turning Equipment | Front end loader in order to load and unload the tunnels. Automatic filling system |
| Temperature during hygienisation | Standard 1: 55°C; Standard 2: 60°C |
| Temperature Monitoring | Temperature should be monitored to ensure that it is representative of the temperatures within the composting mass |
3.2. Full Description of the Material to be treated
3.2.1. Full Description of the Material to be treated as provided by the Applicant
The description of the material to be treated, presented in the current section, has been extracted from the application and has been edited for clarity and abridged in places for brevity.
The feedstock materials to be composted are wastes which are typically found in household food waste collection and commercial premises with the same characteristics. In Directive (EU) 851/2018, the Waste Framework Directive, the definition for this type of waste is:
‘“bio‐waste” means biodegradable garden and park waste, food and kitchen waste from households, offices, restaurants, wholesale, canteens, caterers and retail premises and comparable waste from food processing plants’.
Under the ABP regulations, this waste would be defined as:
Animal by‐products referred to in Article 10 (p) of Regulation (EU) No 1069/2009, i.e. catering waste other than as referred to in Article 8(f) of Regulation (EU) No 1069/2009. Catering waste ‘means all waste food including used cooking oil originating in restaurants, catering facilities and kitchens, including commercial kitchens and household kitchens’.
Animal by‐products referred to in Article 10(f) of Regulation (EU) No 1069/2009 (i.e. products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arise), which have undergone processing as defined in Article 2(1)(m) of Regulation (EC) No 852/2004.
Non‐ABP Material
Some household catering waste collection schemes will also include grass clippings/small branches. In addition, structural bulking materials, such as wood chips, straw and wood shavings, are used to create porosity. These larger woody materials typically do not break down as fast as other non‐woody materials and can persist through to the end of the composting process. They are typically removed from the finished compost at the end of the process with the use of a screen. These screening overs (rejects) can then be reused in the composting process and introduced into new batches of compost as a structural bulking material and as an (microbial) inoculant.
3.2.2. Assessment of the BIOHAZ Panel on the Material to be Treated
The raw materials to be processed by the two proposed transformation standards for composting in a tunnel include catering waste and processed foodstuffs of animal origin no longer intended for human consumption. The assessment exclusively focuses on ABP Cat. 3 materials as described in Article 10 of Regulation (EU) No 1069 of 2009. Article 10 (p) describes Cat. 3 catering waste as food waste other than catering waste (originating) from means of transport operating internationally. Derogation (X), from point 1 Section 2, Chapter III, Annex V of Commission Regulation (EU) No 142/2011 describes products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arise, which have been further processed as per Article 2(1)(m) of Regulation (EU) No 852/2004.
It is important to highlight that the assessment does not address biodegradable garden and park waste included in the definition of biowaste reported in the Directive (EU) 2018/851 amending Directive 2008/98/EC on waste.
A risk assessment (Gale, 2002) on the use of composting and biogas treatments to dispose of catering waste containing meat, conducted by the UK Department for Environment, Food and Rural Affairs (Defra), used data on the composition of household waste showing that uncooked meat accounted for around 1% of the total weight of average household waste. A more recent risk assessment conducted by the UK Waste and Resources Action Programme (WRAP 2017) used estimates of percentages of uncooked meat discarded to waste and going to compost of 2.8% (poultry), 1.39% (pig meat), 0.8% (beef) and 1.09% (lamb). Therefore, it is considered that the material to be treated can contain uncooked or undercooked meat and bones.
3.3. Hazard Identification
3.3.1. Hazard Identification as provided by the Applicant
The hazard identification, presented in the current section, has been extracted from the application and has been edited for clarity and abridged in places for brevity.
The hazards to be addressed are ‘biological ‐ animal/human pathogens’. The pathogens to consider are viruses, bacteria and parasites.
Attention must be drawn to the fact that this application is for catering waste and foodstuffs of animal origin which were intended for human consumption. There are many controls in place with this material because it was intended for people to eat it. In comparison with other feedstocks, such as manure, this feedstock should be deemed low risk.
The UK Department for Environment, Food and Rural Affairs (Defra) conducted a comprehensive analysis nearly 20 years ago of the microbial risks from composting catering waste (Gale, 2002). More recent research by Kohler (2017) was conducted by the German Quality Assurance Organisation for Compost (BGK) of six different household food waste collection services in which the waste was screened to determine what pathogens were present in the raw untreated food waste from households. Based on these reports and a review of the recent occurrence of these pathogens, pathogens were subdivided into two groups:
Pathogens that may enter the composting process, and
Pathogens that are unlikely to enter the composting process.
The six pathogens identified as a risk and that may enter the composting process are: Toxoplasma, Campylobacter, Escherichia coli, Salmonella, Listeria and Clostridium perfringens. Table A.2 (in the annex) gives an overview of the properties of these pathogens that may enter the composting process.
Table A.2.
Pathogens that may enter the composting process
| Organism | Where does it come from | Potential consequence (disease description) | Where does it occur | What is the relationship with compost | References |
|---|---|---|---|---|---|
| Toxoplasma | A parasite that infects vertebrates including birds. Domestic and feral cats are the definitive hosts, but other mammals, including humans, can be infected. | Toxoplasmosis in pregnant women, infection which can lead to mental retardation and loss of vision in their congenitally infected children. | Through the ingestion of undercooked meat, or by ingestion of the oocysts from soil contaminated with cat faeces. | Cat faeces might be disposed of in the household food waste bin. | Nichols (2000) |
| Campylobacter | It may occur in the guts of animals, especially poultry. | Campylobacteriosis, Guillain‐Barre syndrome, reactive arthritis and post infectious irritable bowel syndrome. | Unwashed and uncooked root crops. |
Chicken is discarded uncooked in the catering waste bin. This organism does not grow outside a mammalian or avian host and this may reduce the risk of disease transmission via compost. |
Macklin et al. (2008); Berry et al. (2013). Jones and Martin (2003) Hakkinen et al. (2007) |
| Escherichia coli ( E. coli ) | Lives in the intestines of humans, chickens and other animals. | Depends on the toxins they produce. Symptoms of E. coli infection include diarrhoea, stomach cramps and vomiting. | Associated with contaminated manure or with manure‐contaminated irrigation water. | E. coli can enter the composting process via contaminated material. | (Singh et al., 2010, 2011; Singh, 2011; Jiang et al. (2003); Berry et al. (2013) |
| Salmonella | Lives in the intestines of the chicken but can occur also in other animals. | Causes diarrhoea, abdominal cramps and fever, usually within 12–72 h after infection. | Lives in the intestinal tracts of humans and other animals | Can enter the composting process via contaminated material. Also, there is a possibility of re‐contaminating the compost after the heat phase. |
Macklin et al. (2008) Singh et al. (2010). |
| Listeria | Humans presumably acquire listeriosis from direct contact with infected animals, but several recent outbreaks have confirmed an indirect transmission from animals to humans through consumption of contaminated food products | Listeriosis, flu‐like symptoms, vomiting, diarrhoea, meningitis, septicaemia, spontaneous abortions. | Contaminated food products, including raw milk, pasteurised milk, chocolate milk, butter, soft cheeses, and processed meat and poultry products, have been implicated as sources of human listeriosis cases. |
Inadequately pasteurised compost could be spread on land used in vegetable growing. Contaminated food products sent for composting. |
Nightingale et al. (2004) Vivant et al. (2013) |
| Clostridium perfringens |
Illness appears 8–24 h following ingestion of large numbers of vegetative cells in temperature‐abused protein foods, typically meat and poultry. |
Cause of food‐borne illness, though cases are widely under‐reported because of the mild nature of the gastrointestinal illness, which consists of diarrhoea and abdominal cramps. |
Cells sporulate in the small intestine, producing an enterotoxin. | Meat products will be found in catering waste which is sent for composting. | Labbé and Juneja (2013) |
The pathogens which are unlikely to enter the composting process, according to the applicant, are: Scrapie agents, BSE agents, Foot and Mouth Disease virus, Classical Swine Fever virus, African Swine Fever virus, Swine Vesicular disease virus, Newcastle Disease virus, Clostridium botulinum and Trichinella spiralis.
3.3.2. Assessment of the BIOHAZ Panel on Hazard Identification
The approach taken by the applicant was to provide a list of biological hazards which may enter the composting process (Toxoplasma, Campylobacter, E. coli, Salmonella, L. monocytogenes and Clostridium perfringens) and a list of biological hazards that are unlikely to enter the composting process (Scrapie and BSE agents, the viruses causing Food and Mouth Disease, Classical Swine Fever, African Swine Fever, Swine Vesicular Disease and Newcastle Disease, Clostridium botulinum and Trichinella spiralis).
The applicant based their hazard identification on two studies conducted by the UK Department for Environment, Food and Rural Affairs (Defra) and the German Quality Assurance Organisation for Compost (BGK), published in Gale (2002) and Kohler (2017), respectively.
It is important to highlight that the risk assessment on the use of composting and biogas treatment to dispose of catering waste containing meat conducted by Gale (2002) was exclusively focused on TSE agents, some pig viruses (Foot and Mouth Disease virus, Classical Swine Fever virus, African Swine Fever virus, Aujeszky′s Disease virus), Escherichia coli O157, Campylobacter, Salmonella, the Newcastle disease agent and parasites (Cryptosporidium and Toxoplasma), while Kohler (2017) selectively studied raw untreated food waste from six different household food waste collection services regarding their toxicological (with a focus on Clostridium botulinum toxins) and bacteriological (with a focus on bacterial pathogens) status. The assessment by Gale (2002) has been recently updated by WRAP (the UK Waste and Resources Action Programme) with new information and data. The report by WRAP includes an assessment on the risk to livestock deriving from the composting of catering wastes containing meat (WRAP, 2017), providing a quantitative risk assessment for Foot and Mouth Disease virus, Classical Swine Fever virus, African Swine Fever virus, Swine Vesicular disease virus, Newcastle Disease virus, Toxoplasma gondii, classical and atypical scrapie and BSE. It also includes an overview of risks from other hazards, including Highly Pathogenic Avian Influenza virus and Clostridium botulinum.
The BIOHAZ Panel agrees with the applicant in that Toxoplasma, Campylobacter, pathogenic E. coli, Salmonella, Listeria monocytogenes and Clostridium perfringens are relevant pathogens to be considered when assessing the ability of the proposed alternative method to reduce the risk. However, other pathogenic sporulating and non‐sporulating bacteria (e.g. Clostridioides difficile, Staphylococcus aureus, Enterococcus faecalis, E. faecium) and some viruses are also important hazards, which should also be taken into account.
With regard to viruses, the examples mentioned by the applicant as unlikely to enter the composting process (Foot and Mouth Disease virus, Classical Swine Fever virus, African Swine Fever virus, Swine Vesicular Disease virus and Newcastle Disease virus (Gale, 2002)), and some others, such as porcine circovirus and parvovirus (WRAP,2017) and, based on tissue distribution during infection, Chicken Anaemia virus and some avian reoviruses (EFSA BIOHAZ Panel, 2015, Castaño et al., 2019; Pitcovski and Goyal, 2019) can occur in food waste (particularly in waste material containing uncooked or undercooked meat and bones). Depending on vaccination status, some of these viruses may also occur in animals at or proximate to slaughtering (Brochu et al., 2019; Miłek et al., 2019). Occurrence in uncooked or undercooked meat is also of particular relevance in relation to the proposed association between contaminated food waste and the spread of some viruses, such as the African Swine Fever virus (Gibbens, 2017). The Defra assessment also concluded that multiple composting barriers, including a grazing ban of 2 months were important factors to reduce the risk posed to animal health by some viruses, such as the Classical Swine Fever virus. Other composting barriers include the exclusion of meat at source, the composting hygienisation process, the stockpiling or storage of compost and the decay and dilution of compost in soil (Gale, 2002). Porcine circovirus and parvovirus are considered to be the most heat resistant (Lund et al., 1996; Emmoth et al., 2004; Fröschle et al., 2015), although gyrovirus (Chicken Anaemia virus) also displays a considerable degree of thermoresistance (Welch et al., 2006).
In relation to porcine parvoviruses, the WRAP assessment states that in acute phases of infection, they are shed in faeces, which could contaminate pork meat, and that high levels of viral DNA have been detected in heart, liver and kidneys of infected piglets (WRAP, 2017). Similarly, there is clear evidence that porcine circovirus is present in pig tissues, including muscle and bone marrow. Although the risks of transmission of porcine parvovirus and circovirus through catering waste may be lower than through other routes, it cannot be concluded that the risks are negligible.
Among the hazards identified by the applicant, the most heat resistant non‐sporulating bacteria is considered to be S. Senftenberg 775W, which is the strain of Salmonella enterica with the highest thermal resistance. In addition, Enterococcus (mainly some E. faecium strains) is commonly also considered to be an appropriate surrogate for non‐sporulating bacteria to validate thermal treatments, given its high intrinsic heat resistance (Ma et al., 2007; Smelt and Brul, 2014; Hu and Gurtler, 2017; Brar and Daryluk, 2018; Liu et al., 2018). Spore‐forming bacteria, such as C. perfringens, C. botulinum or C. difficile, present a much higher heat resistance and, therefore, would not be sufficiently reduced either by the conditions proposed or by the approved method (Bhunia, 2018). However, Gale (2002) concluded that the spore levels predicted in compost are no higher than those reported for some soils.
3.4. Level of Risk Reduction
3.4.1. Level of Risk Reduction as provided by the Applicant
The level of risk reduction, presented in the current section, has been extracted from the application and edited for clarity and abridged in places for brevity.
The pathogens identified that may enter the compost system were studied based on available published literature. The temperature and time conditions required for their inactivation or their D values are presented in Table A.3 (in the annex). According to the applicant, these data demonstrate that, in principle, the ECN proposed standards of 55°C for 72 h and 60°C for 48 h are of a sufficient standard to inactivate the pathogens likely to enter the composting process.
Table A.3.
D‐values and inactivation conditions for pathogens that may enter the composting system
| Organism | D‐value | Inactivation Conditions | References | ||
|---|---|---|---|---|---|
| Temp °C | Time | Temp °C | Time | ||
| Toxoplasma | |||||
| Toxoplasma gondii oocysts in water under laboratory conditions | 55 | 2 min | Dubey (1998) | ||
| Toxoplasma gondii oocysts in water under laboratory conditions | 60 | 1 min | Dubey (1998) | ||
| T. gondii tissue cysts in meat under laboratory conditions | 60 | 4 min | Dubey et al. (1990) | ||
| T. gondii tissue cysts in experimentally infected sheep muscles | 60 | 10 min | El‐Nawawi et al. (2008) | ||
| Campylobacter | |||||
| Campylobacter jejuni in agri wastes in laboratory scale digester | 55 | 0.99 min | Ugwuanyl et al. (1999) | ||
| Campylobacter jejuni in agri wastes in laboratory scale digester | 60 | 0.71 min | Ugwuanyl et al. (1999) | ||
| Campylobacter jejuni heated in meat | 60 | 20 seconds | Doyle and Schoeni (1986) | ||
| Escherichia coli | |||||
| Bench scale cow manure composting | 45 | 48 h | Lung et al. (2001) | ||
| E. coli 0157: H7 in fresh dairy compost with 50% moisture content | 50 | 72 h | Singh et al. (2011) | ||
| E. coli 0157: H7 in fresh dairy compost with 50% moisture content | 55 | 48 h | Singh et al. (2011) | ||
| E. coli 0157: H7 in unautoclaved manure compost | 55 | 35.4 min | Jiang et al.(2003) | ||
| E. coli 0157: H7 in autoclaved manure compost | 55 | 50.3 min | Jiang et al. (2003) | ||
| E. coli 0157: H7 in autoclaved manure compost | 55 | 3 h | Jiang et al. (2003) | ||
| E. coli NCTC 9001 in sludge | 55 | 2.13 min | Lang and Smith (2008) | ||
| E. coli O157: H7 in manure‐based mushroom compost substrate | 54.4 | 8 h | Weil et al. (2013) | ||
| E. coli 0157: H7 in unautoclaved manure compost | 60 | 3.9 min | Jiang et al. (2003) | ||
| E. coli 0157: H7 in autoclaved manure compost | 60 | 4.1 min | Jiang et al. (2003) | ||
| E. coli 0157: H7 in autoclaved manure compost – inactivated | 55 | 15 min | Jiang et al. (2003) | ||
| E. coli 0157: H7 in manure compost | 65 | 3.9 min | Jiang et al. (2003) | ||
| E. coli 0157: H7 in fresh dairy compost with 50% moisture content | 60 | 24 h | Singh et al. (2011) | ||
| Listeria | |||||
| Listeria monocytogenes in ground beef roast | 54.4 | 22.4 min | Schoeni et al. (1991) | ||
| Listeria monocytogenes in ground beef roast | 57.2 | 15.7 min | |||
| Listeria monocytogenes in ground beef roast | 60 | 4.47 min | |||
| Listeria monocytogenes in ground beef roast | 62.8 | 2.56 min | |||
| Listeria monocytogenes in mushroom growth compost substrate | 54.5 | 8 h | Weil et al. (2013) | ||
| Listeria monocytogenes in mushroom growth compost substrate | 60 | 30 min | Weil et al. (2013) | ||
| Listeria monocytogenes in ready‐to‐eat chicken‐fried beef patties | 55 | 81.37 min | Osaili et al. (2006) | ||
| Listeria monocytogenes in ready‐to‐eat chicken‐fried beef patties | 60 | 22.98 min | Osaili et al. (2006) | ||
| Listeria monocytogenes in compost | 55 | 6 h | Singh et al. (2010) | ||
| Listeria monocytogenes in compost | 60 | 70 min | Singh et al. (2010) | ||
| Clostridium perfringens | |||||
| Clostridium perfringens enterotoxin | 60 | 5 min | Naik and Duncan (1977) | ||
| Clostridium perfringens vegetative cells in pork luncheon roll | 55 | 16.3 min | Byrne et al. (2006) | ||
| Rapid death of vegetative cells at 51.6°C, no recovery 24 h later,’Complete inhibition of growth occurring at 49–52°C’ | 51.6 | 24 h | Hall and Angelotti (1965) | ||
| Clostridium perfringens vegetative cells in beef | 55 | 21.6 min | Juneja and Marmer (1998) | ||
| Clostridium perfringens vegetative cells in beef | 60 | 5.3 min | |||
| Clostridium perfringens vegetative cells in turkey | 55 | 17.5 min | |||
| Clostridium perfringens vegetative cells in turkey | 62.5 | 1.3 min | |||
| Six Strains of Clostridium perfringens – little or no growth at 55°C | 55 | Rey et al. (1975) | |||
| Salmonella | |||||
| Salmonella Senftenberg 775W in liquid manure | 50 | 56.7 min | Soldierer and Strauch (1991) | ||
| Salmonella in cattle manure | 50 | 18 h | Singh et al. (2010) | ||
| Salmonella spp. in poultry compost with 50% moisture content | 50 | 96 h | Singh (2011) | ||
| Salmonella Senftenberg 775W | 50.5 | 11.7 h | Elving (2012) | ||
| Salmonella Senftenberg 775W in saline solution | 49 | 26 h | Elving (2009) | ||
| Salmonella Senftenberg in fresh manure | 49 | 100 h | |||
| Salmonella Senftenberg 775W in saline solution | 52 | 8.3 h | |||
| Salmonella Senftenberg in fresh manure | 52 | 17.2 h | |||
| Salmonella Senftenberg 775W in saline solution | 55 | 4.5 h | |||
| Salmonella Senftenberg in fresh manure | 55 | 16.9 h | |||
| Salmonella Senftenberg 775W in meat, 100% moisture | 55 | 36 min | Ceustermans et al. (2006) | ||
| Salmonella Senftenberg 775W in meat, 60% moisture | 55 | 104 min | Ceustermans et al. (2006) | ||
| Salmonella Senftenberg 775W in liquid manure | 55 | 11.5 min | Soldierer and Strauch (1991) | ||
| Salmonella Senftenberg 775W in sludge | 55 | 3.2 min | Lang and Smith (2008) | ||
| Salmonella in cattle manure | 55 | 4 h | Singh et al. (2010) | ||
| Salmonella Senftenberg 775W | 55 | 89 min | Burge et al. (1987) | ||
| Salmonella Senftenberg 775W in meat under lab scale composting trials | 60 | 10 h | Ceustermans et al. (2006) | ||
| Salmonella – composting trial of biowaste | 60 | 10 h | Ceustermans et al. (2006) | ||
| Salmonella‐composting of biowastes in tunnels at the DDSVerko composting plant in Belgium | 60 | 10 h | Ceustermans et al. (2006) | ||
| Salmonella spp. in poultry compost with 50% moisture content | 60 | 24 h | Singh (2011) | ||
| Salmonella Senftenberg 775W in liquid manure | 60 | 2.3 min | Soldierer and Strauch (1991) | ||
| Salmonella in manure‐based mushroom compost substrate | 60 | 30 min | Weil et al. (2013) | ||
| Salmonella Senftenberg 775W | 60 | 7.5 min | Burge et al. (1987) | ||
| Salmonella in cattle manure | 60 | 10 min | Singh et al. (2010) | ||
| Salmonella Senftenberg 775W in saline solution | 70 | 15 min | Elving (2009) | ||
| Salmonella Senftenberg 775W | 70 | 5 min | Elving (2012) | ||
According to the applicant, the ECN proposal of 55°C for 72 h is supported by other researchers; Droffner and Brinton (1995) suggested that at least 3 days at 55°C are needed for sufficient pathogen inactivation and Burge et al. (1987) stated that a minimum temperature of 55°C for 2.5 days is required to reach a level acceptable for commercial products from compost.
Although this application deals with category 3 material (catering waste and processed foodstuffs of animal origin), there is some work conducted which supports the ECN proposal of 55°C by Elving (2009) on higher risk category 2 material manure. It was found that the thermal treatment of fresh manure at 55°C, over 16.9 h, was sufficient to achieve a 5 log10 reduction in Salmonella Senftenberg and Enterococcus spp. For pathogen inactivation at a lower temperature, an increased time is needed to reach the statutory requirements. Elving (2009) indicated that a time of 17.2 h at 52°C or 16.9 h at 55°C can be sufficient to reach the reduction targets set by European Communities (EC) legislation based on the inactivation of Enterococcus spp. in fresh cattle manure. This interval would also be sufficient for a 5 log10 reduction in Salmonella Senftenberg 775W.
Plant Seed Sanitisation
Tomato seeds are a resilient seed to sanitise. Although this application to EFSA is in relation to animal and human pathogens, the applicant considered that work done by Idelmann (2006) on plant seeds supports the proposed standards in this application. In this study by Idelmann, it was shown that there is a three‐factor relationship between the time required to render tomato seeds non‐viable, the temperature level of the treatment and the moisture content of the tomato seeds. It was recognised that this relationship could be used to develop a sanitisation equation, which can be used to prove the sanitisation of compost based on temperature data. This proof of sanitisation is based on the principle that the heat tolerance of tomato seeds can be increased by decreasing the moisture content of the seeds. This equates the heat tolerance of tomato seeds to the heat tolerance of pathogens and weed seeds. The assessment of sanitisation is based on the assumption that eradication of pathogens is gained when total loss of germination ability of the tomato seeds, representing the heat tolerance of most resistant pathogens and weed seeds, is achieved.
The sanitation equation was used to calculate the level of inactivation expected. At a temperature of 55°C, an exposure time of 13.4 h was estimated to have an equal level of sanitisation compared to a temperature of 70°C and an exposure time of 1 h. At a temperature of 60°C, an exposure time of 5.6 h was estimated to have an equal level of sanitisation compared to a temperature of 70°C and an exposure time of 1 h.
Validation Reports of Some Composting Plants in Different EU Countries
Table 1 outlines the findings of some validation studies carried out at commercial scale composting plants in Belgium, the Netherlands, Portugal and the United Kingdom, where validation was carried out as part of an authorisation process carried out by the relevant competent authority in each member state, in accordance with the ‘alternative validation’ procedure provided for in Annex V, Chapter 3, Section 2 of Regulation (EU) No 142/2011.
The plants listed in Table 1 demonstrated the overall reduction of bacterial hazards requested in Annex V, Chapter 3, Section 2 of Regulation (EU) No 142/2011 and were approved to operate.
Portugal
In 2015, Intermunicipal Waste Management of Greater Porto (LIPOR) developed a study to demonstrate that their composting plant was operating in accordance with the requirements of the EU ABP regulations.
During the experiment, a spiked culture containing a high concentration (approximately 108 CFU ml−1) of an indicator organism, Enterococcus faecalis strain ATCC 29212, was used. The analysis of experimental results concluded that, for a multi‐tunnel system such as LIPOR's composting plant and the same mixing input, a period of exposure of 24 h and a temperature of 60°C ensured the sanitation conditions required under the guidelines applicable to animal by‐products. The experimental results showed a reduction of more than 7 log10 cycles for E. faecalis.
Similarly, the Lipor plant tunnel No 12 (Table 1) demonstrated that a standard with the same time/temperature regime as the ECN proposed standard number 2 (60°C for 48 h at 200 mm particle size), albeit at 150 mm particle size, does demonstrate the required log reduction of pathogens to be an approved plant.
Belgium/Flanders
OVAM (Public Waste Agency of Flanders) did a study in 2018 where three different composting plants with different systems were validated according to the procedure in Annex V, Chapter 3, Section 2 of Regulation (EU) No 142/2011. For tunnel composting, the tunnels were validated for working at 60°C for 24 h and 55°C for 48 h, and this showed that a decrease of > 7 log10 of Enterococcus faecalis was achieved.
The Netherlands
The Dutch Waste Management Association commissioned a national study in 2006 aimed at determining the microbiological status of the sector in light of the ABP Regulation (EU) No 1774/2002. During the 2006 study, 21 Dutch composting plants were assessed to determine if they could meet the EU ABP requirements (Tables 1 and 2). Overall, the 21 plants demonstrated a 4.7 log unit reduction for Enterococcus (7.1 down to 2.4). Fifteen of the 21 plants showed a reduction of almost 5 log units or more and met the ABP requirements. The trials in the 21 plants were conducted as follows:
Untreated biowaste was tested for Enterococcus.
After the sanitation phase, the compost was sampled to show a log reduction.
Table 2.
Data from Dutch composting plants as provided by the applicant as additional information
| Number | Plant name | Composting System | Time (Days) | Temperature (°C) | Log reductions Enterococcus (log10) |
|---|---|---|---|---|---|
| 1 | Maastricht | Tunnel | 1 | 60 | 5.5 |
| 2 | Venlo | Tunnel | 1 | 60 | 5.54 |
| 3 | Deurne | Tunnel | 1 | 60 | 5.65 |
| 4 | Moerdijk | Closed Hall | 7 | 55–65 | 4.93 |
| 5 | Nieuwdorp | Closed Hall | 7 | 55–65 | 6.47 |
| 6 | Europoort | Tunnel | 1 | 60 | 4.91 |
| 7 | Bergschenhoek | Tunnel | 1 | 60 | 5.53 |
| 8 | R'dam Botlek | Closed Hall | 7 | 55–65 | 2.99 |
| 9 | Duiven | Closed Hall | 7 | 55–65 | 5.8 |
| 10 | Wilp | Open air composting | 14 | 55 | 3.32 |
| 11 | Alphen a/d Rijn | Closed Hall | 7 | 55‐65 | 4.2 |
| 12 | Rijssenhout | Open air composting | 14 | 55 | 2.5 |
| 13 | Hengelo | Tunnel | 1 | 60 | 4.85 |
| 14 | Lelystad | Tunnel | 1 | 60 | 3.52 |
| 15 | Lelystad Biocel | anaerobic digestion followed by tunnel composting | 15 | 35–40 | 4.8 |
| 16 | Drachten | Closed Hall | 7 | 55–65 | 3.42 |
| 17 | Wijster | Closed Hall | 7 | 55–65 | 6.54 |
| 18 | Usquert | Closed Hall | 7 | 55–65 | 5.85 |
| 19 | Middenmeer | Closed Hall | 7 | 55–65 | 6.33 |
| 20 | Purmerend | Closed Hall | 7 | 55–65 | 3.88 |
| 21 | Oude Pekela | Tunnel | 1 | 60 | 3.36 |
The untreated biowaste samples of all 21 plants had almost the same level of Enterococcus.
United Kingdom
In the United Kingdom, in 2009, the composting plant Envar got approval for a new alternative transformation standard (60°C for 48 h with a maximum particle size of 40 cm, in a tunnel) for composting catering waste from its national Competent Authority – the Animal & Plant Health Agency (APHA).
ECN Standards Particle Size Justification
The ECN is proposing for both standards a maximum particle size of ABP feedstock of 200 mm before entering the tunnel.
The reported maximum particle size of collected biowaste/municipal solid waste from households is in the region of 100 mm (Nakamura et al., 2006; Lakshmikanthan et al., 2014). It should be noted that in some Member States (France, Germany, Slovenia and Austria), National Standards for processing catering waste have no limits on the particle size.
In determining different time–temperature profiles for ABP materials information was gathered on:
The time of inactivation of different types of animal pathogens at different temperatures (presented above).
Information on heat conduction in compost particles, e.g. how long will it take for temperature to reach the core of the compost aggregates as a function of aggregate size and temperature. This information is obtained from data on heat transfer coefficients and heat capacity, which is used for theoretical calculations.
Heat Penetration in a Compost Particle/Aggregate
Compost consists of individual solid particles and aggregates (conglomeration of individual particles and water) of a certain size. As no air enters these aggregates, no aerobic degradation and self‐heating takes place inside this particle/aggregate. The temperature within the core of these particles/aggregates can only increase by heat conduction from the surrounding warmer air and material. In other words, it takes time for the core of the particle/aggregate to reach the same temperature as the temperature at which the composting process is controlled.
The heat conduction of the material depends on its properties (thermal conductivity, heat capacity and density), and moreover, the time for the temperature to reach the core of the particle/aggregate depends on the size of the particle. The properties of the material measured for different types of composting materials were reviewed from the following publications:
-
–
Study of thermal conductivity in organic solid wastes before composting (Huet et al., 2012a).
-
–
The impact of compaction, moisture content, particle size and type of bulking agent on initial physical properties of sludge‐bulking agent mixtures before composting (Huet et al., 2012b).
-
–
Determination of thermal properties of composting bulking materials (Ahn et al., 2009).
-
–
Testing of thermal properties of compost from municipal waste with a view to using it as a renewable, low temperature heat source (Klejment and Rosiński, 2008).
Models are available in the food processing industry to calculate heat penetration in food to determine the required time to pasteurise and sterilise food in cans. A model (Rouweler, 2014) was used to calculate the core temperature of a particle/aggregate in warm air as a function of the material properties and the size. The model can be used for different geometries (sphere, oval, brick, cylinder, cube, etc.). Figure A.1 in the annex shows the temperature development in the core of a sphere‐shaped particle/aggregate in time as a function of the particle diameter, as predicted through modelling. The initial particle temperature is 20°C and the temperature of composting is 60°C.
Figure A.1.
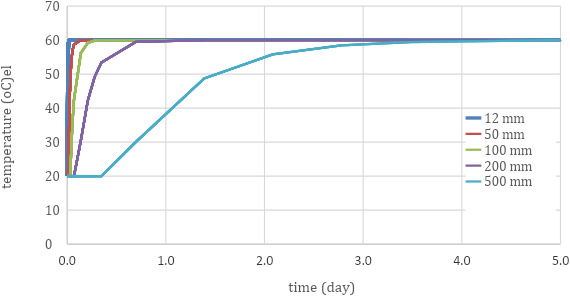
Time for Particles of Different Diameter Sizes to Reach 60°C (using a model developed for heat treatment of foods (Rouweler, 2014))
As it can be seen, the time to reach the target temperature increases significantly when the particles get larger. If we want a time–temperature profile of 2 days at 60°C for pathogen eradication, the particles should be smaller than 200 mm, or otherwise it takes too long for the temperature to reach a temperature of 60°C in the core of the particles.
Figure A.2, in the annex, shows that the time for a particle with a 200 mm diameter to reach 60°C is 1.39 days (33.36 h). Idelmann (unpublished) also set up a trial in an open windrow composting process (Figures A.3 and A.4, in the annex) and investigated the time lag of heat penetration into wood, which typically has a low heat conductivity. The trial determined that the time for the centre of the wood to reach 56°C was less than 10 h.
Figure A.2.
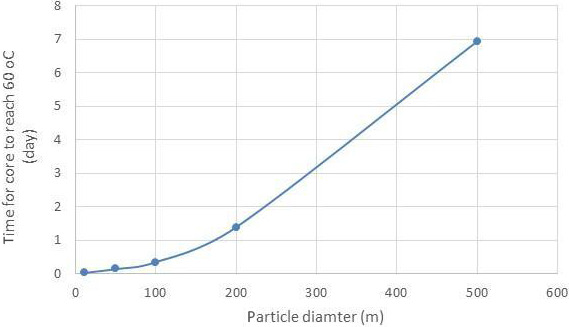
The time for a particle with a 200 mm diameter to reach 60°C is 1.39 days (33.36 h) (using a model developed for heat treatment of foods (Rouweler, 2014))
Figure A.3.
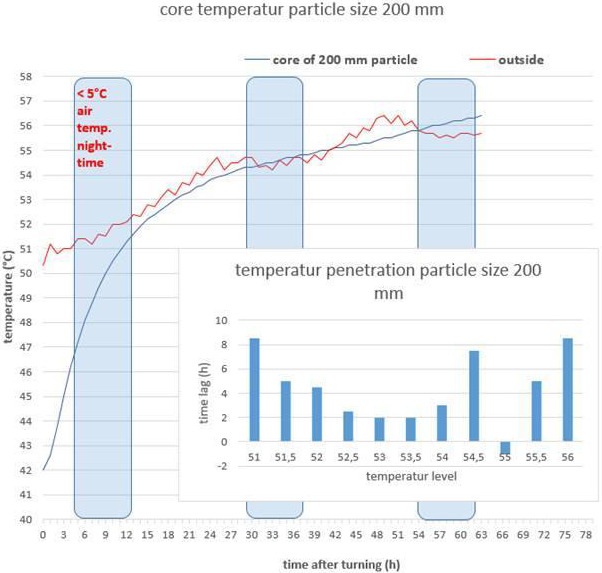
Time for Temperature to reach 56°C in wood in a composting process (Idelmann, unpublished)
Figure A.4.

Photograph of the trial of the wood with a temperature probe in the centre (Idelmann, unpublished)
3.4.2. Assessment of the BIOHAZ Panel on the Risk Reduction
The applicant did not perform any validation experiment with direct measurements of the reduction of viability/infectivity of endogenous indicator or spiked surrogate microorganisms and/or viruses, but provided as supporting information (i) data from the literature on heat resistance and D‐values of the listed biological hazards which, according to the applicant, may contaminate the raw materials to be composted; (ii) treatment temperature/time equivalence calculations based on establishing a relationship between the heat tolerance of tomato seeds and the heat tolerance of pathogenic microorganisms; (iii) the findings of some validation studies carried out at commercial scale composting plants across Europe, where validation of similar alternative composting methods was carried out in accordance with the ‘alternative validation’ procedure provided for in Annex V, Chapter 3, Section 2 of Regulation (EU) No 142/2011; (iv) modelling and experimental data based on the penetration of heat in packaged food and composted wood.
The data gathered by the applicant on heat resistance and D‐values of Toxoplasma, Campylobacter, Escherichia coli, Listeria monocytogenes, Clostridium perfringens and Salmonella come from experimental studies carried out in a range of different matrices. In this regard, it is well known that microbial heat resistance is influenced by the composition and physico‐chemical characteristics (e.g. moisture content) of the heated medium, as well as by other factors such as the bacterial physiological status (Smelt and Brul, 2014) and, therefore, extrapolations made from heat resistance data obtained in a different matrix to the compost scenario may not be representative. Most information available in the literature on the heat resistance of the relevant biological hazards for the methods under assessment have been obtained in laboratory media or in foods. The information available in the literature on heat resistance and D‐values of bacterial hazards during composting processes comes from studies conducted in simulated waste model systems or using manure as a starting material. These studies described, for Enterococcus faecalis, D60‐values and D55‐values ranging from 4.72 to 5.24 h and from 6.61 to 8.30 h, respectively (Ugwuanyl et al., 1999). With these heat resistance parameters, treatments of at least 23.6 h at 60°C or 41.5 h at 55°C should achieve a 5 log10 reduction of E. faecalis. For generic Enterococcus spp., with D55‐values ranging from 0.34 to 11.0 h (Elving, 2009, 2012), a treatment of at least 55 h at 55°C would provide the required 5 log10 reduction. The thermal tolerance of other microorganisms belonging to the Enterobacteriaceae is well recognised to be lower than that of Enterococcus spp. For Salmonella Senftenberg 775W, the Salmonella strain with the greatest reported thermal tolerance (Ng et al., 1969), D55‐values ranging from 0.31 to 6.2 h have been reported (Elving, 2009, 2012). Thus, a treatment for at least 31 h at 55°C would allow reaching the required 5 log10 reduction. Considering a z‐value of 5.3°C (Elving, 2012) for this Salmonella strain, a treatment for at least 3.53 h at 60°C would achieve the required 5 log10 reduction. Other results have been reported in the literature with different D‐ and/or z‐values in other substrates or food systems (Doyle and Mazzotta, 2000; Elving,2009, 2012), but they also allowed to predict a reduction of 5 log10 or higher for the time/temperature combinations proposed by the applicant. Therefore, it can be concluded that the proposed treatment standards, if the target temperature–time combinations are maintained during the whole composting process and applied homogeneously in the composting tunnel, would be able to inactivate more than 5 log10 of E. faecalis or S. Senftenberg 775W in the material to be treated, as required in Section 2, Chapter III, Annex V, of Regulation (EU) No 142/2011.
The treatment temperature/time equivalence calculations in tomato seeds were based on the assumption that eradication of pathogens is achieved when total loss of germination ability of the tomato seeds is achieved and on experimental data in this regard published by Idelmann (2006). The control of weeds is considered by several countries and, in particular, tomato seeds are included in the German national regulation (JRC, 2014). The temperature dependence of heat inactivation parameters is specific for the organism under study and the heating medium. It is classically defined by its z‐value (temperature increase needed to cause a 10‐fold decrease in the D‐value). Thus, while the D‐value (time required at a constant temperature to achieve a 1 log (or 90%) reduction of the organism) at a given temperature and in a given matrix can be the same for two different organisms (e.g. Salmonella and respective moisture adjusted tomato seeds), it is not demonstrated whether z‐values are similar. The apparent similar thermal inactivation values for a given bacteria and plant seed may be coincidental or only apply under a specific set or parameters (pH. water activity, matrix etc.). Therefore, an extrapolation from the behaviour of tomato seeds to that of biological hazards cannot be considered valid.
The validation studies carried out at commercial scale composting plants across Europe (Table 1) showed in most cases log10 reductions for Enterococcus faecalis exceeding the 5 log10 required in Annex V, Chapter 3, Section 2 of Regulation (EU) No 142/2011. Only one of those validation studies monitored log10 reductions for Salmonella Senftenberg and a > 7 log10 decrease in this microorganism was observed, also exceeding the 5 log10 reductions. None of the validation studies evaluated the level of reduction of thermoresistant viruses, such as parvovirus. A validation plan to achieve at least a 3 log10 reduction of infectivity titre of parvovirus and a 5 log10 reduction of S. Senftenberg was provided by the applicant from ENVAR, a plant in the UK, but neither the detailed description of the study nor the results were provided, and therefore, it is not possible to evaluate its efficacy. Moreover, in some of the validation studies provided as supporting information, the process method (type of composting) and process parameters (temperature, time or particle size) are different from the alternative transformation parameters of the processes under assessment. Also, in approximately half of the compost plants tested in the Dutch validation studies (Table 2), supplied as additional information, the 5 log10 reduction of Enterococcus was not achieved, which shows that, in practice, variability in the accomplishment of microbial reduction targets does occur.
The heat penetration experiment in composted wood carried out by Idelmann shows that for particle sizes of 200 mm, it can take around 24 h to reach the composting temperature. The results of heat penetration modelling, using the methodology described in Rouweler (2014) for estimating heat penetration and pasteurisation times of packaged foods, show that it takes between approximately 18 h (Figure A.1, in the annex) and 33.36 h (Figure A.2, in the annex) for a particle of 200 mm to reach 60°C. It is uncertain how applicable this model is to the composting process, especially in an industrial context. The time needed to reach the processing temperatures in the larger particles (200 mm) should not be considered as part of the composting processing times proposed in the alternative methods.
The applicant did not provide any supporting information demonstrating the capacity of the proposed transformation standards to inactivate viruses, including thermoresistant viruses.
3.5. HACCP Plan
3.5.1. HACCP Plan as provided by the Applicant
The description of the HACCP plan presented in the current section has been extracted from the application and edited for clarity and abridged in places for brevity.
Introduction
A generic HACCP plan was designed to assess the risks in a composting plant scenario which had the proposed two ABP transformation standards (Tables 3, 4 and 5).
Table 3.
Description of the Compost Product
| Composition | Category 3 ABP materials |
|---|---|
| Structure and Physical–chemical properties | The material is a semi‐solid material with a water content of less than 40% |
| Processing |
Standard 1: (a) maximum particle size of ABP before entering the tunnel: 200 mm; (b) minimum temperature in all material in the tunnel unit: 55°C; and (c) minimum exposure time in the tunnel unit without interruption: 72 h Standard 2: (a) maximum particle size of ABP before entering the tunnel: 200 mm; (b) minimum temperature in all material in the tunnel unit: 60°C; and (c) minimum exposure time in the tunnel unit without interruption: 48 h |
| Packaging | Some sold in bulk trailer loads and some in bags |
| Storage Conditions | It will be stored in a clean area separate from the dirty area |
| Shelf‐life | Not applicable |
| Instructions for use | It will be used on agricultural land, landscaping, growing media and horticulture |
| Microbiological Criteria |
Samples of compost are taken after hygienisation for E. coli and samples of compost are taken from the plant (during storage) prior to dispatch for Salmonella. Escherichia coli: n = 5, c = 1, m = 1,000 cfu/g, M = 5,000 cfu/g; Salmonella: absence in 25 g; n = 5; c = 0 |
Table 4.
List of hazards, controls & corrective actions
| Process Step | Hazard | Control | Corrective Action |
|---|---|---|---|
| 1. Waste intake | The presence of pathogens (other than those mentioned in Table A.2) from wrong type of ABP waste allowed into the plant |
Prerequisite programme 1: ‘feedstock acceptance form’ Supplier approval in advance by the Feedstock Approval Contract Supplier commercial document (if applicable) Visual inspection of solid waste by operator |
Review acceptability of load Non‐conforming material is rejected Review suitability of suppliers |
| 2. Storage |
Pathogens. Untreated ABP waste could contaminate finished compost |
Untreated waste kept in a separate area from finished compost | Re‐training of staff |
| 3. Mixing & Blending of ABP materials to less than 200 mm particle size | Survival of pathogens after hygienisation due to incorrect size of ABP feedstock |
Pretreatment Training of staff Visual check by operators and taking of random samples to pass ABP feedstock through 200 mm mesh screen |
Failed material is re‐blended Re‐training of staff |
| 4. Hygienisation of feedstock | Survival of E. coli/pathogens due to incorrect hygienisation (under‐processed) |
Consistent application of the scheduled process (temperature and time) Checking and calibrating the thermograph Perquisite programmes of planned maintenance and calibration of temperature probes Trained staff Check mixes Mixing system Procedure for failure of hygienisation |
If the compost fails to reach the required heat treatment, the material is reprocessed again The cause of the problem is investigated and appropriate action taken to ensure an effective process |
| 5. Composting/curing process | Microbial pathogens could re‐contaminate the compost |
Separate areas Trained staff Cleaning and disinfection of material when used in both the clean and dirty area No use of leachate water (PERCOLATE) after required time/temp has been reached |
Re‐training of staff If compost is re‐contaminated, it will be reprocessed |
| 6. Dispatch of compost |
Microbial pathogens could re‐contaminate the compost Biosecurity: dissemination of hazards to local farms and the environment |
Laboratory analysis of compost for Salmonella |
If the compost has Salmonella present the veterinary officer is contacted for instructions on what to do The cause of the problem is investigated and appropriate action taken to ensure an effective process |
Table 5.
List of Hazards, Controls and Determination of CCPs
| Process Step | Hazard | Control | Campden Tree | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q2a | Q3 | Q4 | Q5 | CCP? | |||
| 1. Waste intake | The presence of pathogens from wrong type of ABP waste allowed into the compost plant |
Prerequisite programme (PRP): ‘feedstock acceptance form’ Supplier approval in advance by the Feedstock Approval Contract Supplier commercial document – if applicable Visual inspection of solid waste by operator |
Yes |
Not a CCP Operational PRP as it is an important PRP 1 |
|||||
| 2. Storage |
Pathogens. The facility operates separate dirty and clean areas to prevent cross‐contamination |
Only dirty loader in dirty area or if used in clean area: procedure and registration of cleaning and disinfection of loader | Yes |
Not a CCP, managed by PRP 2 |
|||||
| 3. Mixing/shredding all feedstocks to less than 200 mm particle size | Survival of pathogens at hygienisation due to incorrect size of feedstock | Visual inspection to ensure less than 200 mm of ABP feedstock | Yes |
Not a CCP, managed by PRP 2 |
|||||
| 4 Hygienisation of feedstock | Survival of E. coli due to incorrect hygienisation (under‐processed) |
PRP – Consistent application of the scheduled process (temperature and time) Prerequisite programmes of planned maintenance and calibration of temperature probes Trained staff |
No | Yes | Yes | Yes, CCP1 | |||
| 5. Composting/curing process | Microbial pathogens could re‐contaminate the compost |
PRP Use of clean loader |
Yes | Not a CCP | |||||
| 6. Dispatch of compost | Microbial pathogens could re‐contaminate the compost |
PRP Use of Clean loader Salmonella testing |
Yes | Not a CCP | |||||
The HACCP plan was drawn up based on the HACCP principles and includes the seven HACCP steps.
Prerequisite programmes
The plant must have in place a number of prerequisite programmes including:
Feedstock acceptance procedures
Procedures in relation to transformation parameters achievement
Hygienisation procedures
Material sampling procedures
Microbial failure procedures
Cleaning and hygiene procedures
Procedures to prevent recontamination of post‐hygienisation material and compost, respectively
Vermin and pest control procedures
Maintenance and calibration procedures.
Dispatch procedures
Procedures required in order to implement the HACCP plan effectively – HACCP Audit
Training
Relevant Regulations
The HACCP plan was developed in compliance with:
Regulation (EU) No 1069 of 2009 and Regulation (EU) No 142 of 2011.
Code of Practice
The following codes of practice/guidelines were followed:
The document ‘Guidance Document Implementation of procedures based on the HACCP principles, and facilitation of the implementation of the HACCP principles in certain food businesses’
The BRI Campden HACCP intermediate training course manual.
A decision tree based on the Campden BRI Guideline Document, which was used to assess if a hazard was a critical control point.
Hazard Analysis
Microbial Hazards
Based on the feedstocks going to be used in the compost plant, the relevant pathogen hazards are listed in Section C – Hazards.
Identification of intended use
The compost will be used on agricultural land, landscaping projects and in horticultural uses.
Listing of hazards, control measures and determination of CCPs
3.5.2. Assessment of the BIOHAZ Panel on the HACCP plan
A generic HACCP plan was provided by the applicant with reference to a composting plant scenario in which the two proposed ABP transformation standards are applied. The HACCP plan was developed by a multidisciplinary team to cover the entire composting process, from raw material intake to dispatch of the finished compost to the end users.
The biological hazards identified by the applicant as a risk for the composting process are listed in Section C of the dossier.
Figure 2 summarises the process flow diagram and by‐products and Table 4 lists the process steps as in Figure 2. However, in Figure 2, step 5 is named ‘Post sanitisation treatment and screening’, while in Table 4, it is called ‘compositing/curing process’. Moreover, in Figure 2, step 6 is named ‘passed E. coli, Salmonella and temperature standard – dispatched to end users’ while in Table 4, it is called ‘Dispatch of compost’.
Figure 2.
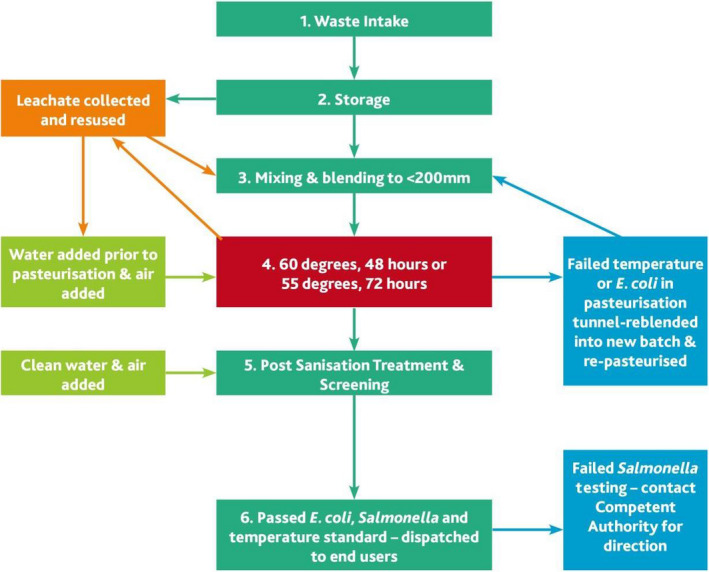
Process Flow and By‐products (as provided by the applicant)
In both Figure 2 and Table 4, step 2 is storage but it specifically refers to storage of untreated waste. A further storage step should be added before the step described as dispatch of compost.
Indeed, in the description of the material flow in the composting process, at page 6 of the dossier, the applicant states that the compost is moved with a clean loader to avoid cross‐contamination to further processing by screening. Screening is done to remove impurities. This is done in a separate area from the raw feedstock to prevent cross‐contamination of pathogens.
The Critical Control Points (CCPs) were identified following the Campden BSI decision tree (Gaze, 2015).
Step 1 – waste intake was not identified as a CCP in relation to the presence in the feedstock of additional pathogens in comparison to those listed in Section C and this is considered to be correct. Feedstock suppliers sign a contract describing the waste material which can be provided.
Step 2 – storage was not identified as a CCP and this is considered to be correct. However, the identified hazard was cross‐contamination between untreated and finished compost while this hazard applies only to the storage step after the hygienisation and composting process, not included in the flow diagram. A potential hazard that might occur during storage before processing is the proliferation of the pathogens identified in section C, leading to substantial increases in numbers. Therefore, an indicative storage time and temperature should be included.
Step 3 – mixing and blending of ABP materials to less than 200 mm particle size was not identified as a CCP and this is considered to be correct. As specified by the applicant, if the expected particle size is not achieved, pathogens listed in section C might survive after hygienisation. However, this feedstock preparation phase should enable the achievement of the expected particle size as well as the water content (50–65%) specified in the technical data. The process efficacy is verified by taking random samples tested through a 200 mm mesh screen. It is important to highlight that (1) thermal properties of compost bulking materials change according to particle size but also according to water content and bulk density (Ahn et al., 2009); (2) at present in some member states (i.e. France, Germany, Slovenia and Austria), national standards for processing catering waste have no limits on the particle size. According to the applicant, water is added when needed to maintain an adequate water content, but it is not specified how and when this is controlled.
Step 4 – Hygienisation of feedstock is identified as a CCP and this is considered to be correct because, as stated by the applicant, if the process is not performed at the appropriate temperature and water content for the appropriate time the pathogens listed in section C might survive. The applicant did not specify if installations for monitoring temperature over time would be used along with recording devices to register, where appropriate continuously and in the coldest spot of the tunnel, the results of the monitoring measurements referred to time and temperature. As this step is a CCP, quantitative values to be verified to guarantee that the process is under control should be added as well as means of verification and recording systems.
Step 5 – The composting/curing process is not identified as a CCP and this is considered to be correct. Indeed, the only identified hazard is cross‐contamination of the processed compost, but this can be avoided by keeping the treated compost in a dedicated area which must be different to that used for the storage of untreated ABP waste and using separated instruments for transport. The compost/curing process described in Tables 3 and 4 seems to correspond to the step described as Post‐sanitisation treatment and screening in Figure 2. This step contributes to the pathogen eradication because thermophilic process conditions providing a temperature range > 55°C are kept between 10 days to several weeks. The applicant did not clarify if this is always the case whatever the season or weather conditions and did not identify this step as a key and relevant barrier for pathogens inactivation. The applicant refers to the cleaning and disinfection of material when used in both clean and dirty areas, while separate equipment should be used.
Step 6 – Dispatch of compost is not identified as a CCP and this is considered to be correct. As above, the only identified hazard is cross‐contamination of the processed compost which can be avoided by keeping the treated compost in a dedicated area well separated from the dirty area. The laboratory analysis of Salmonella cannot be considered as a validation of the control measure, but a means of verification.
As the only reference of the approved method by the ABP Regulation (EU) No 1069/2009 was a hygienisation provision for compost by direct methods of Escherichia coli or Enterococcaceae for process verification (< 1,000 CFU/g in four of five samples; 1,000–5,000 CFU/g in one of five samples) and for Salmonella in the final compost, which should not be detected (in 25 g) in five of five samples, it is considered that an alternative process should comply with those requirements from a hygienic point of view.
3.6. Risk Associated with Interdependent Processes
3.6.1. Risk Associated with Interdependent Processes as Provided by the Applicant
The level of risk associated with interdependent processes, presented in the current section, has been extracted from the application and edited for clarity and abridged in places for brevity.
Leachate from the Process
Leachate collected from the composting tunnels and wash water used to clean trucks/floor/machines in the reception hall is typically used in the composting process prior to hygienisation. This leachate should be stored separately from clean water. Procedures should be in place to ensure that no unpasteurised/dirty water is used in the process after the minimum hygienisation temperature of 60°C is maintained for at least 48 h or 55°C for 72 h, as it carries a risk of reintroducing pathogens if used.
Storage
The end product compost (organic fertiliser and/or soil improver) should be stored in an area of the compost plant where there is no possibility of cross‐contamination with raw unprocessed animal by‐products. This will ensure there is no reintroduction of pathogens.
3.6.2. Assessment of the BIOHAZ Panel on the Risk associated with interdependent Processes
The applicant provided a description of the risks associated with leachate from the process and storage of raw materials and the end product, as well as the procedures that would be implemented for dealing with these risks. Transport of the end product should be considered.
3.7. Risk associated with the Intended End Use of the Product
3.7.1. Risk associated with the Intended End Use of the Product as provided by the Applicant
The level of risk associated with the intended use of the product, presented in the current section, has been extracted from the application and edited for clarity and abridged in places for brevity.
Once the compost end product meets all the proposed transformation standard requirements, and meets the required pathogen thresholds, there will be no risks associated with the end use of the product. This application recommends to define the end point in the manufacturing chain at the composting plant once the compost product is in compliance with the microbial testing requirements in the ABP regulation.
3.7.2. Assessment of the BIOHAZ Panel on the Risk associated with the Intended End Use of the Product
The end product of the process is compost, which according to the applicant may be used as a fertiliser and/or soil improver (it will be used on agricultural land, for landscaping projects and for horticultural uses). The applicant envisages the establishment of the end point of the process at the composting plant when the end product complies with microbial testing standards. Provided that the alternative method is capable of achieving a risk reduction level equivalent to that of the method in the Regulation and that these microbial standards are met, no additional risks associated with the intended end use of the product are foreseen.
4. Conclusions
The materials to be composted by the two proposed transformation standards for hygienisation in tunnel composting include ABP catering waste and processed foodstuffs, which were intended for human consumption, and other non‐ABP material (i.e. garden and park waste). The assessment of the BIOHAZ Panel exclusively focuses on the ABP raw materials catering waste and processed foodstuffs intended for human consumption.
The applicant provided a list of biological hazards which may enter the composting process (Toxoplasma, Campylobacter, pathogenic E. coli, Salmonella, Listeria monocytogenes and Clostridium perfringens). However, other pathogenic bacteria, such as Clostridioides difficile, Staphylococcus aureus, Enterococcus faecalis or E. faecium, and viruses, including thermoresistant viruses such as porcine parvovirus and circovirus or chicken anaemia virus, are important hazards which should also be taken into account, especially as uncooked or undercooked meat may be present in household and catering waste.
Taking into account the criteria set out in Annex V Chapter 3 Section 2 of Regulation (EU) 142/2011, the EFSA BIOHAZ Panel considered that a reduction of 5 log10 of Enterococcus faecalis or Salmonella Senftenberg 775W, and a 3 log10 reduction of the most relevant thermoresistant viruses (such as parvovirus) should be demonstrated to validate the alternative methods.
The applicant did not provide any validation experiments with direct measurements of the reduction of viability of endogenous indicators or spiked surrogate bacteria. However, from thermal inactivation parameters reported in the literature, it can be concluded that the proposed composting standards can achieve at least a 5 log10 reduction of Enterococcus faecalis or Salmonella Senftenberg 775W.
The applicant did not consider thermoresistant viruses as a relevant hazard and therefore did not provide any data from direct measurements of the reduction of infectivity of spiked thermoresistant viruses, nor provide data from validation studies undertaken at national level or data from literature supporting the efficacy of the proposed composting standards on thermoresistant viruses. However, thermoresistant viruses should be considered to be a relevant hazard in this context and validation data should have been provided accordingly.
The BIOHAZ Panel considers that the generic HACCP plan provided and the information about the risks of the interdependent processes and those associated with the intended end use are generally appropriate and can be the basis for the validation and verification of the process once implemented at an industrial level.
The BIOHAZ Panel considers that the evidence provided by the applicant does not demonstrate that the requirements of Annex V, Chapter 3, Section 2 of Commission Regulation (EU) No 142/2011 are achieved.
Documentation as provided to EFSA
Evaluation of alternative methods of tunnel composting. 1st submission September 2019, 2nd submission November 2019, submission of additional information in May 2020. Submitted by the European Composting Network (ECN).
Abbreviations
- ABP
animal by‐products
- APHA
the Animal & Plant Health Agency (United Kingdom)
- BGK
German Quality Assurance Organisation for Compost
- BIOHAZ
EFSA Panel on Biological Hazards
- CA
Competent Authority
- Cat.
Category
- Defra
Department of the Environment Food and Rural Affairs (United Kingdom)
- HACCP
hazard analysis critical control point
- WRAP
the UK Waste and Resources Action Programme
Annex – Information provided by the applicant in support of the evaluation
1.
The tables and figures in the annex were copied verbatim from the application.
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Bolton DJ, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman LM, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Escámez PF, Ortiz‐Peláez A, Ashe S and Alvarez‐Ordóñez A, 2020. Scientific Opinion on the Evaluation of Alternative Methods of Tunnel Composting (submitted by the European Composting Network). EFSA Journal 2020;18(8):6226, 36 pp. 10.2903/j.efsa.2020.6226
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00583
Panel members: Ana Allende, Avelino Alvarez‐Ordóñez, Declan Joseph Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Maria Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Acknowledgements: The Panel wishes to thank Katrine Bote for her contributions to the drafting of this scientific output.
Adopted: 8 July 2020
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figures 1, 2, A.1 and A.2: © as provided by the applicant, ECN; Figures A.3 and A.4: © Idelmann (unpublished).
References
- Ahn HK, Sauer TJ, Richard TL and Glanville TD, 2009. Determination of thermal properties of composting bulking materials. Bioresource Technology, 100, 3974–3981. [DOI] [PubMed] [Google Scholar]
- Berry ED, Millner PD, Wells JE, Kalchayan N and Guerini MN, 2013. Fate of Naturally Occurring Escherichia coli O157:H7 and Other Zoonotic Pathogens during Minimally Managed Bovine Feedlot Manure Composting Processes. Journal of Food Protection, 76, 1308–1321. [DOI] [PubMed] [Google Scholar]
- Bhunia AK, 2018. Foodborne Microbial Pathogens. Chapter, 12.
- Brar PK and Daryluk MD, 2018Validation of Enterococcus faecium as a surrogate for Salmonella under different processing conditions for peanuts and pecans. Food Microbiology, 80, 9–17. [DOI] [PubMed] [Google Scholar]
- Brochu NM, Guerin MT, Varga C, Lillie BN, Brash ML and Susta L, 2019. A two‐year prospective study of small poultry flocks in Ontario, Canada, part 1: prevalence of viral and bacterial pathogens. Journal of Veterinary Diagnostic Investigation, 31, 327–335. 10.1177/1040638719843577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge WD, Enkiri NK and Hussong D, 1987. Salmonella regrowth in compost as influenced by substrate. Microbial Ecology, 14, 243–253. [DOI] [PubMed] [Google Scholar]
- Byrne B, Dunne G and Bolton DJ, 2006. Thermal Inactivation of Bacillus cereus and Clostridium perfringens vegetative cells and spores in pork luncheon roll. Food Microbiology, 23, 803–808. [DOI] [PubMed] [Google Scholar]
- Castaño P, Benavides J, Fernández M, Fuertes M, Royo M, Pérez V, Ferreras MC, Lee MS and Fernández JM, 2019. Tissue Tropism of Chicken Anaemia Virus in Naturally Infected Broiler Chickens. Journal of Comparative Pathology, 167, 32–40. [DOI] [PubMed] [Google Scholar]
- Ceustermans A, De Clercq D, Aertsen A, Michiels C, Geeraerd A, Van Impe J, Coosemans J and Ryckeboer J, 2006. Inactivation of Salmonella Senftenberg strain W775 during composting of biowastes and garden wastes. Journal Applied Microbiology, 103, 53–64. [DOI] [PubMed] [Google Scholar]
- Doyle ME and Mazzotta AS, 2000. Review of studies on the thermal resistance of Salmonellae. Journal of Food Protection, 63, 779–795. 10.4315/0362-028x-63.6.779 [DOI] [PubMed] [Google Scholar]
- Doyle MP and Schoeni JL, 1986. Isolation of Campylobacter jejui from retail mushrooms. Applied and Environmental Microbiology, 51, 449–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droffner ML and Brinton WF, 1995. Survival of E. coli and Salmonella populations in aerobic thermophilic composts as measured with DNA gene probes. Zentralblatt für Hygiene und Umweltmedizin, 197, 387–397. [PubMed] [Google Scholar]
- Dubey J, 1998. Toxoplasma gondii oocyst survival under defined temperatures. Journal of Parasitology, 84, 862–865. [PubMed] [Google Scholar]
- Dubey JP, Hartley WJ, Lindsay DS and Topper MJ, 1990. Fatal congenital Neospora caninum infection in a lamb. Journal of Parasitology, 76, 127–130. [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010. Statement on technical assistance on the format for applications for new alternative methods for animal by‐products. EFSA Journal 2010;8(7):1680, 12 pp. 10.2903/j.efsa.2010.1680 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2015. Risk to public and/or animal health of the treatment of dead‐in‐shell chicks (Category 2 material) to be used as raw material for the production of biogas or compost with Category 3 approved method. EFSA Journal 2015;13(11):4306, 46 pp. 10.2903/j.efsa.2015.4306 [DOI] [Google Scholar]
- El‐Nawawi F, Tawfik M and Shaapan R, 2008. Methods for inactivation of Toxoplasma gondii cysts in meat and tissues of experimentally infected sheep. Foodborne Pathogens and Disease, 5, 687–690. [DOI] [PubMed] [Google Scholar]
- Elving J, 2009. Pathogen inactivation and regrowth in organic waste during biological treatment. Licentiate Thesis, Department of Biomedical Sciences and Veterinary Public Health Swedish. University of Agricultural Sciences, Uppsala. [Google Scholar]
- Elving J, 2012. Thermal treatment of organic waste and its function as a controlled risk mitigation strategy. [Google Scholar]
- Emmoth E, Dergel I, McNeilly F, Allan G, Albihn A and Klingeborn B, 2004. Heat Inactivation of Porcine Circovirus Type 2
- Fröschle B, Heiermann M, Lebuhn M, Messelhäusser U and Plöchl M, 2015. Hygiene and Sanitation in Biogas Plants. In: Biogas Science and Technology Edited by Georg Gübitz, Alexander Bauer, Guenther Bochmann, Andreas Gronauer, Stefan Weiss. Springer, 2015.
- Gale P, 2002. Risk Assessment: Use of Composting and Biogas Treatment to Dispose of Catering Waste Containing Meat. Department for Environment, Food and Rural Affairs (Defra), UK. [Google Scholar]
- Gaze R, 2015. HACCP: A practical guide 5th edn 2015 (Campden BRI). Guideline G42 ISBN 978‐0-907503‐82-8.
- Gibbens N, 2017. African swine fever and the risks of feeding food waste to pigs. Vet Rec., 181, 642. [PubMed] [Google Scholar]
- Hakkinen M, Heiska H and Hänninen ML, 2007. Prevalence of Campylobacter spp. in cattle in Finland and antimicrobial susceptibilities of bovine Campylobacter jejuni strains. Applied & Environmental Microbiology, 73, 3232–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall HE and Angelotti R, 1965. Clostridium perfringens in meat and meat products. Applied Microbiology, 13, 3562–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M and Gurtler JB, 2017Use in Food Safety Challenge Studies: A Review. Journal of Food Protection, 80, 1506–1536. 10.4315/0362-028x.jfp-16-536 [DOI] [PubMed] [Google Scholar]
- Huet J, Druilhe C and Debenest G, 2012a. Study of thermal conductivity in organic solid wastes before composting. 8th International Conference ORBIT 2012, Jun 2012, Rennes, France. 8 p. ffhal‐00732437. Available online: https://hal.archives-ouvertes.fr/hal-00732437/document
- Huet J, Druilhe C, Tremier A, Benoist JC and Debenest G, 2012b. The impact of compaction, moisture content, particle size and type of bulking agent on initial physical properties of sludge‐bulking agent mixtures before composting. Bioresource Technology, 114, 428–436. [DOI] [PubMed] [Google Scholar]
- Idelmann M, 2006. Hygienisierung von Kompost ‐ Möglichkeiten zum Nachweis einer erfolgreichen Abtötung von Pathogenen und Unkrautsamen. [Hygienisation of compost ‐ Possibilities to prove successful eradication of pathogens and weed seeds.]. Kassel University Press. [Google Scholar]
- Jiang X, Morgan J and Doyle MP, 2003. Thermal inactivation of Escherichia coli O157:H7 in cow manure compost. Journal Food Protection., 66, 1771–1777. [DOI] [PubMed] [Google Scholar]
- Jones P and Martin M, 2003. A Review of the Literature on the Occurrence and Survival of Pathogens of Animals and Humans in Green Compost. The Waste and Resources Action Programme. ISBN: 1‐84405‐063-7
- JRC , 2014. End‐of-waste criteria for biodegradable waste subjected to biological treatment (compost & digestate): Technical proposals. JRC Scientific and Policy Reports 2014.
- Juneja VK and Marmer BS, 1998. Thermal inactivation of Clostridium perfringens vegetative cells in ground beef and turkey as affected by sodium pyrophosphate. Food Microbiology, 15, 281–287. [Google Scholar]
- Klejment E and Rosiński M, 2008. Testing of thermal properties of compost from municipal waste with a view to using it as a renewable, low temperature heat source. Bioresource Technology, 99:8850–8855.41. [DOI] [PubMed] [Google Scholar]
- Kohler B, 2017. Quantitiative bakteriologische und toxikologische Untersuchung von 6 Biogutproben unter besonderer Berucksichtgung von Salmonellen sowie Clostrdium botulinum und Botulinum‐Neurotoxin. [Quantitative bacteriological and toxicological investigation of food samples with special attention to Clostridium botulinum and botulinum toxin.]
- Lakshmikanthan P, Santthosh LG and Babu G, 2014. Evaluation of bioreactor landfill as sustainable land disposal method 10.13140/2.1.1182.9763 [DOI] [Google Scholar]
- Lang NL and Smith SR, 2008. Time and temperature inactivation kinetics of enteric bacteria relevant to sewage sludge treatment processes for agricultural use. Water Research, 42, 2229–2241. [DOI] [PubMed] [Google Scholar]
- Liu S, Tang J, Tadapaneni RK, Yang R and Zhu MJ, 2018. Exponentially Increased Thermal Resistance of Salmonella spp. and Enterococcus faecium at Reduced Water Activity. Applied and Environment Microbiology, 84, e02742–17. 10.1128/AEM.02742-17 Published 2018 Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B, Jensen VF, Have P, Ahring B, 1996. Inactivation of virus during anaerobic digestion of manure in laboratory scale biogas reactors. Antonie van Leeuwenhoek, 69, 25–31. 10.1007/BF00641608 [DOI] [PubMed] [Google Scholar]
- Lung AJ, Lin CM, Kim JM, Marshall MR, Norstedt R and Thompson NP, 2001. Destruction of Escherichia coli O157:H7 and Salmonella enteritidis in cow manure composting. Journal of Food Protection, 64, 1309–1314. [DOI] [PubMed] [Google Scholar]
- Ma L, Kornacki JL, Zhang G, Lin CM and Doyle MP, 2007. Development of Thermal Surrogate Microorganisms in Ground Beef for In‐Plant Critical Control Point Validation Studies. Journal of Food Protection, 70, 952–957. [DOI] [PubMed] [Google Scholar]
- Macklin KS, Hess JB and Bilgili SF, 2008. In‐house windrow composting and its effects on foodborne pathogens The . Journal of Applied Poultry Research, 17, 121–127. [Google Scholar]
- Miłek D, Woźniak A, Guzowska M and Stadejek T, 2019. Detection Patterns of Porcine Parvovirus (PPV) and Novel Porcine Parvoviruses 2 through 6 (PPV2‐PPV6) in Polish Swine Farms. Viruses, 11, 474 Published 2019 May 24. 10.3390/v11050474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik H and Duncan C, 1977. Thermal Inactivation of Clostridium perfringens Enterotoxin . Journal of Food Protection, 4I, 100–103. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Castaldi MJ and Themelis NJ, 2006. Measurement of Particle Size and Shape of New York City Municipal Solid Waste and Combustion Residues Using Image Analysis.
- Ng H, Bayne HG and Garibaldi JA, 1969. Heat resistance of Salmonella: the uniqueness of Salmonella senftenberg 775W. Applied Microbiology, 17, 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols GL, 2000. Food‐borne protozoa. British Medical Bulletin, 55, 209–235. [DOI] [PubMed] [Google Scholar]
- Nightingale KK, Schukken YH, Nightingale CR, Fortes ED, Ho AJ, Her Z, Grohn YT, Mc Donough PL and Wiedmann M, 2004. Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environment. Applied & Environmental Microbiology, 4458–4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaili T, Griffis CL, Martin EM, Beard BL, Keener A and Marcy JA, 2006. Thermal inactivation studies of Escherichia coli 0157:H7, Salmonella, and Listeria monocytogenes in ready to eat chicken fried beef patties. Journal of Food Protection, 69, 1080–1086. [DOI] [PubMed] [Google Scholar]
- Pitcovski J and Goyal SM, 2019. Avian Reovirus Infections Chapter 11 Nov 2019. 10.1002/9781119371199.ch11 [DOI]
- Rey CR, Walker HW and Rohrbaugh PL, 1975. The influence of temperature on growth, sporulation, and heat resistance of spores of six strains of Clostridium perfringens . Journal of Milk & Food Technology, 38, 461–465. [Google Scholar]
- Rouweler W, 2014. How to Estimate the Heat Penetration Factors fh and fc, and the Heating Lag Factors jh and jc, required for Calculating the Sterilization or Pasteurization Times of Packaged Foods. Available online: https://www.researchgate.net/publication/262426454_How_to_Estimate_the_Heat_Penetration_Factors_fh_and_fc_and_the_Heating_Lag_Factors_jh_and_jc_required_for_Calculating_the_Sterilization_or_Pasteurization_Times_of_Packaged_Foodspdf
- Schoeni J, Brunner K and Doyle MP, 1991. Rates of thermal inactivation of Listeria monocytogenes in beef and fermented beaker sausage. Journal of Food Protection, 54, 334–337. [DOI] [PubMed] [Google Scholar]
- Singh R, 2011. Thermal inactivation of stress adapted pathogens in compost. Clemson University. PhD Dissertation.
- Singh R, Jiang X and Luo F, 2010. Thermal inactivation of heat‐shocked Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes in dairy compost. Journal of Food Protection, 73, 1633–1640. [DOI] [PubMed] [Google Scholar]
- Singh R, Kim J, Shepherd M, Luo F and Jiang X, 2011. Determining thermal inactivation of Escherichia coli O157:H7 in fresh compost by simulating early phases of the composting process. Applied & Environmental Microbiology, 77, 4126–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelt J and Brul S, 2014. Thermal inactivation of microorganisms. Critical Reviews in Food Science and Nutrition. [DOI] [PubMed] [Google Scholar]
- Soldierer W and Strauch D, 1991. Kinetics of the inactivation of Salmonella during thermal disinfection of liquid manure. Zentralblatt für Veterinarmedizin B, 38, 561–574. [PubMed] [Google Scholar]
- Ugwuanyl JO, Harvey LM and McNeil B, 1999. Effect of process temperature, pH and suspended solids content upon pasteurization of a model agricultural waste during thermophilic aerobic digestion. Journal of Applied Microbiology, 87, 387–395. [DOI] [PubMed] [Google Scholar]
- Vivant AL, Garmyn D and Piveteau P, 2013. Listeria monocytogenes, a down‐to‐earth pathogen. Frontiers in Cellular and Infection Microbiology, 3, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil J, Cutter C, Beelman R and Laborde L, 2013. Inactivation of human pathogens during phase II composting of manure‐based mushroom growth substrate. Journal of Food Protection, 76, 1393–1400. [DOI] [PubMed] [Google Scholar]
- Welch J, Bienek C, Gomperts E and Simmonds P, 2006. Resistance of porcine circovirus and chicken anemia virus to virus inactivation procedures used for blood product. The Journal of Transfusion, 46, 1951–1958. [DOI] [PubMed] [Google Scholar]
- WRAP , 2017. Compost quality and safety for agriculture. UK Waste and Resources Action Programme, WRAP, Banbury. [Google Scholar]


