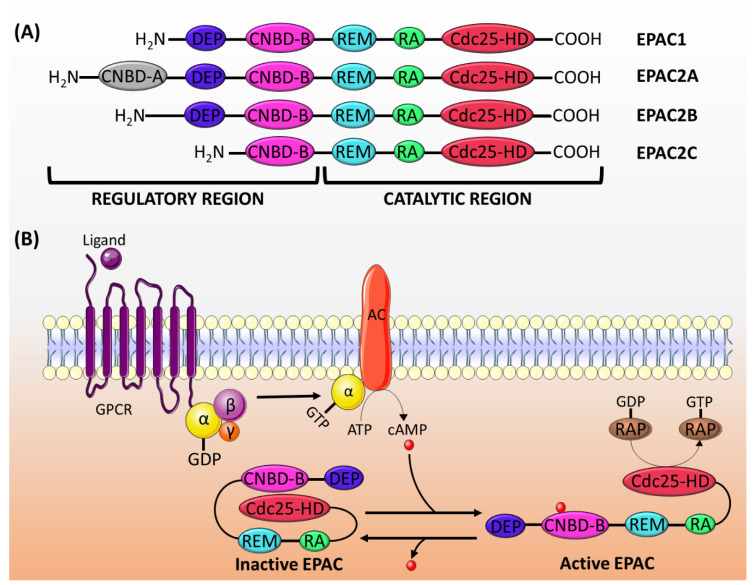
Figure 1.
(A) The structure of EPAC proteins. EPAC is made up of a catalytic region and a regulatory region, each of which is divided into different domains. The catalytic region in all EPAC isoforms comprises three domains: REM, RA and Cdc25-HD. The regulatory region consists of two domains: DEP and CNBD-B. EPAC2A has an addition CNBD-A domain, whereas EPAC2C lacks the DEF domain. (B) The mechanism of EPAC proteins activation: the activation of adenylyl cyclase (AC) by the Gα subunit of Gs protein induces the production of cAMP, which binds to the CNBD-B within the regulatory region of EPAC. This binding induces a conformational change releasing the auto-inhibitory effect, and it permits the binding of Rap1/2 to the catalytic domain (Cdc25-HD) and its subsequent activation by the GEF activity of EPAC. GPCR: G-protein coupled receptor.