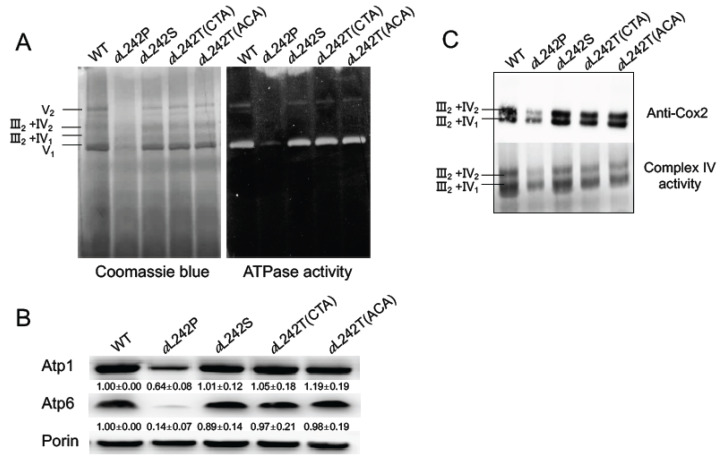
Figure 2.
Assembly of OXPHOS complexes. For these experiments mitochondria were isolated from wild type (WT) and the mutant strains aL242P, aL242T and aL242S grown for 24 h at 28 °C in rich galactose liquid media until a density of 2-3 OD600nm. (A) Mitochondria were solubilized with 2 g of digitonin/g protein, and samples of 250 µg were separated by BN-PAGE in a 3–12% acrylamide gel. In the left panel the proteins are stained with Coomassie blue; in the right panel, F1FO complexes are revealed in-gel by their ATPase activity. (B) Total mitochondrial proteins were resolved by SDS-PAGE analysis (50 µg per lane), transferred to a nitrocellulose membrane and probed with antibodies against the indicated proteins. Values are normalized to porin and expressed relative to WT ± standard deviation. (C) Mitochondria were solubilized with 10 g of digitonin/g protein, and samples of 250 µg were separated as in (A) and then transferred to a PVDF membrane. Oligomers of Complex IV associated to complex III (III2-IV2 and III2-IV1) were probed with antibodies against the Cox2 subunit of Complex IV (upper panel) and in-gel by the activity of Complex IV (lower panel). The shown gels are representative of at least 3 experiments.