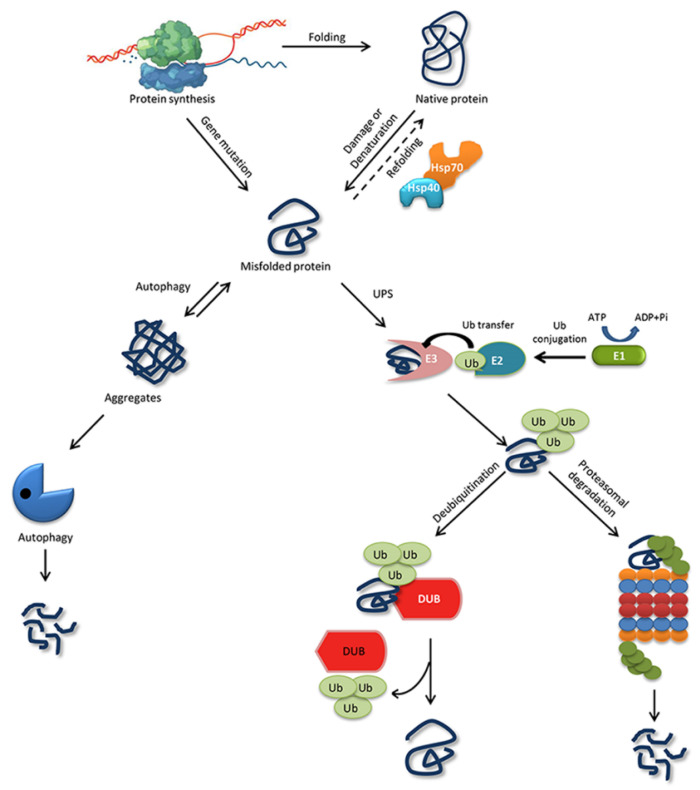
Figure 1.
Protein folding, misfolding, and degradation. Protein folding starts during the ribosomal translation process and attain the native conformation to execute cellular processes. The native folded proteins are often misfolded due to mutations and other environmental factors. Molecular chaperones catalyze the folding/refolding events, disaggregation of the protein aggregates, and targeting the protein for degradation. Aggregates are typically degraded by autophagy, whereas the ubiquitin proteasome system (UPS) degrades the destabilized/misfolded proteins by covalent attachment of a ubiquitin molecule assisted by E1-E2-E3 enzymes. The ubiquitinated proteins are recognized by the 26S proteasome and are degraded. However, the ubiquitin moiety is cleaved off by Deubiquitinating enzymes (DUBs) and the protein can be rescued from the degradation cycle.