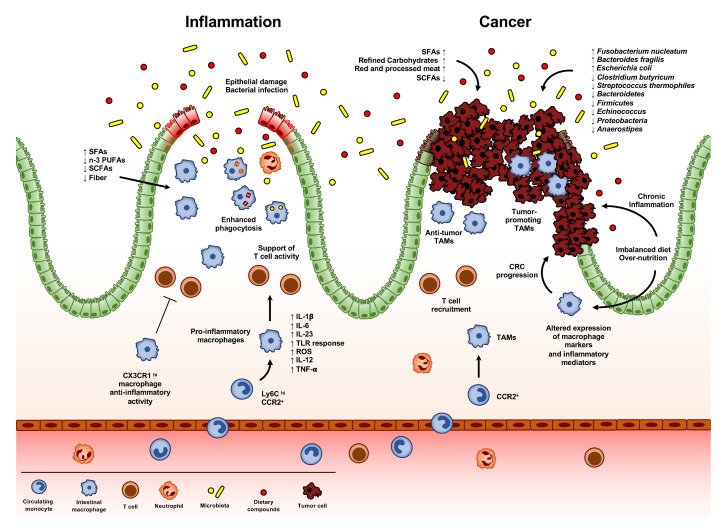
Figure 3.
Intestinal macrophages in inflammation and cancer. Alteration of intestinal immune homeostasis may result in acute or chronic inflammation. This typically occurs during inflammatory bowel disease (IBDs), damage of the epithelial layer, or infection. Dietary components can exert either beneficial or detrimental effects in these processes, activating a pro-inflammatory response in macrophages, like saturated fatty acids (SFAs), or preventing an inflammatory response, like short-chain fatty acids (SCFAs), fibers, or n-3 polyunsaturated fatty acids (n-3 PUFAs). This state of inflammation is characterized by the recruitment and accumulation of Ly6Chi CCR2+ monocytes, which differentiate in pro-inflammatory macrophages, able to secret inflammatory molecules, such as IL-1β, IL-6, IL-23, ROS, IL-12 and TNF-α, to promptly respond to TLR engagement and to support T cell activity. On the other hand, CX3CR1hi macrophages maintain their pro-resolving function. During the resolution of inflammation, intestinal macrophages increase their phagocytic activity, engulfing epithelial apoptotic cells, neutrophils and invading bacteria. The composition of the gut microbiota exerts a crucial role in CRC progression, also impacting on the efficacy or toxicity of many therapeutic agents, including immunotherapies. Differences in the composition of the gut microbiota have been observed between healthy individuals and CRC patients: the presence of bacteria species, such as Fusobacterium nucleatum, Bacteroides fragilis and Escherichia Coli, has been associated with CRC progression. Conversely, other bacteria, including Clostridium butyricum, Streptococcus thermophiles, and the species Bacteroidetes, Firmicutes, Echinococcus, Proteobacteria and Anaerostipes have been negatively associated with the network of butyrate-producing CRC-depleted microbes. In addition, some nutritional constituents, including saturated fatty acids (SFAs), refine carbohydrates and red and processed meat, have pro-inflammatory properties, while short-chain fatty acid (SCFAs) production, as a consequence of microbiota metabolism of dietary fiber, reduces the risk of CRC occurrence, by regulating the intestinal inflammatory response. In addition, overnutrition and imbalanced diet (such as the Western diet), can favor obesity, increasing chronic inflammation and altering the expression of macrophage markers and inflammatory mediators, finally resulting in CRC progression. Tumor-associated macrophages (TAMs) represent the most abundant leukocyte population infiltrating neoplastic tissues. Differently from other tumor types, the role of TAMs in CRC is still controversial. Several studies reported a correlation between higher TAM infiltration and CRC poor prognosis, while other claimed their antitumor activity. The general idea is that the role of TAMs in CRC progression can be influenced by their localization within the tumor tissue: intratumoral macrophages are likely to be more influenced by the tumor cell-generated milieu, thus displaying a protumoral activity; while TAMs localized at the tumor invasive front are less subjected to the conditioning of the tumor-microenvironment, thus exerting anti-tumoral functions. Furthermore, infiltration of T cells at the tumor invasive front has been demonstrated to correlate with better patients’ prognosis.