Abstract
The impact of coronavirus disease‐19 (COVID‐19) in liver recipients remains largely unknown. Most data derive from small retrospective series of patients transplanted years ago. We aimed to report a single‐center case series of five consecutive patients in the early postoperative period of deceased‐donor liver transplantation who developed nosocomial COVID‐19. Two patients presented important respiratory discomfort and eventually died. One was 69 years old and had severe coronary disease. She rapidly worsened after COVID‐19 diagnosis on 9th postoperative day. The other was 67 years old with non‐alcoholic steatohepatitis, who experienced prolonged postoperative course, complicated with cytomegalovirus infection and kidney failure. He was diagnosed on 36th postoperative day and remained on mechanical ventilation for 20 days, ultimately succumbing of secondary bacterial infection. The third, fourth, and fifth patients were diagnosed on 10th, 11th, and 18th postoperative day, respectively, and presented satisfactory clinical evolution. These last two patients were severely immunosuppressed, since one underwent steroid bolus for acute cellular rejection and another also used anti‐thymocyte globulin for treating steroid‐resistant rejection. Our novel experience highlights that COVID‐19 may negatively impact the postoperative course, especially in elder and obese patients with comorbidities, and draws attention to COVID‐19 nosocomial spread in the early postoperative period.
Keywords: COVID‐19, liver transplantation, postoperative period, severe acute respiratory syndrome coronavirus 2
Abbreviations
- ABTO
Brazilian Association of Organ Transplantation
- ACR
acute cellular rejection
- COVID‐19
coronavirus disease‐19
- CT
computed tomography
- DDLT
deceased‐donor liver transplantation
- HCC
hepatocellular carcinoma
- ICU
intensive care unit
- LT
liver transplantation
- NASH
non‐alcoholic steatohepatitis
- NO
naso‐oropharyngeal
- OTI
orotracheal intubation
- PMT
pulse methylprednisolone therapy
- POD
postoperative day
- RT‐PCR
real‐time polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
- TACE
transarterial chemoembolization
1. INTRODUCTION
Brazil is currently one of the most affected countries in the world by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) and has become the epicenter of the coronavirus disease‐19 (COVID‐19) pandemic in Latin America. 1 The impact of COVID‐19 in liver recipients remains largely unknown, as most data derive from case reports and small retrospective series. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Moreover, most of them address patients who were transplanted years ago. 2 , 3 , 4 , 5 , 6 , 8 , 9 , 10 , 12 Information regarding patients in the early postoperative period is very scarce.
Many authors recommend weaning of immunosuppression and some even advocate its complete withdrawn. 2 , 4 , 9 , 12 , 16 Nevertheless, these suggestions are mostly based on patients in late postoperative period, for whom immunosuppression is already diminished and acute cellular rejection (ACR) is less likely. Another distinct feature is the impact of nosocomial infection, as these patients are generally infected in‐hospital. When the pandemic starts its declining phase, community viral spread is expected to decrease; however, nosocomial infection may persist longer and affect more routinely hospitalized patients who just underwent transplantation.
São Paulo is Brazil's largest city and the current epicenter of the disease in the country. Our institution is one of largest transplantation centers in Latin America, having performed more than 3000 liver transplants (LT), with an average of 160 LT per year. The aim of this study was to report a single‐center case series of consecutive patients in the early postoperative period of deceased‐donor liver transplantation (DDLT) who developed COVID‐19.
2. CASE REPORTS
During the first months of the city quarantine (March 24th to May 31st, 2020), we performed 19 DDLT in 18 patients and diagnosed 5 cases of COVID‐19 in the early postoperative period. In all cases, SARS‐CoV‐2 infection was confirmed via real‐time polymerase chain reaction (RT‐PCR) in naso‐oropharyngeal (NO) swab or tracheal secretion. Nucleic acid (RNA) was extracted with an automated method based on magnetic beads (mSample Preparation System RNA, Abbott). Reverse transcription, amplification, and detection were performed following an in‐house protocol validated in the Laboratory Division (College of American Pathologists accredited) comprising an E gene assay as the first‐line screening tool, followed by confirmatory testing with a N gene assay, as previously described. 17 Endogenous gene RNAse P was used as internal control of extraction and amplification, as well as positive and negative external controls. Analytical sensitivity was 40 copies/mL, and specificity in samples containing other respiratory viruses RNA was 100%. Table 1 depicts a summary of all cases, and Table 2 shows laboratory assessment at time of COVID‐19 diagnosis.
Table 1.
Summary of cases with early postoperative COVID‐19
| Case | Age, sex, and BMI | Liver Disease | Comorbidities | Donor data | Surgical data | Immunosuppression protocol | COVID‐19 symptoms | COVID‐19 treatment and outcome |
|---|---|---|---|---|---|---|---|---|
| 1 |
69 y, female BMI: 34.71 kg/m2 |
HCV Downstaged HCC MELD: 15 Child‐Pugh: A6 |
SAH Coronariopathy Pulmonary hypertension |
Female, 45 y BD: hemorrhagic stroke |
GW: 1600 g TST: 285 min TIT: 585 min WIT: 35 min BBP: None |
Basiliximab, tacrolimus, mycophenolate and intraoperative corticoid bolus and tampering |
9th POD Fever Mild dyspnea Diarrhea |
OTI on 12th POD Azithromycin Death on 13th POD due to due to refractory shock and acidosis. |
| 2 |
67 y, male BMI: 32.81 kg/m2 |
NASH α1‐antitripsin deficiency MELD: 13 Child‐Pugh: B7 Hepatic encephalopathy |
SHA Obesity |
Female, 22 y BD: subarachnoid hemorrhage 21 d ICU AST 156 UI/mL ALT 296 UI/mL GGT 584 mg/dL |
GW: 1475 g TST: 435 min TIT: 445 min WIT: 40 min BBP: None |
Basiliximab, tacrolimus, mycophenolate and intraoperative corticoid bolus and tampering |
36th POD Fever Hypoactive Delirium Progressive dyspnea |
OTI on 37th POD Azithromycin Hydroxychloroquine Death on 56th POD due to secondary bacterial infection |
| 3 |
69 y, male BMI: 27.58 kg/m2 |
Alcoholic cirrhosis HCC MELD: 13 Child‐Pugh: A6 |
SHA DM |
Male, 54 y BD: cranioencephalic trauma |
GW: 1370 g TST: 425 min TIT: 405 min WIT: 35 min BBP: None |
Tacrolimus and intraoperative corticoids bolus with tampering |
10th POD Fever watery diarrhea dry cough Mild exertional dyspnea |
Venturi mask Supportive care Dyspnea worsened on 10th hospitalization day Discharged home on 17th hospitalization day |
| 4 |
59 y, male BMI: 24.38 kg/m2 |
Cryptogenic cirrhosis Ascites Hepatic encephalopathy MELD: 10 Child‐Pugh: B7 |
Hepatosplenic schistosomiasis |
Female, 52 y BD: hemorrhagic stroke Presented cardiac arrest before organ recovery |
GW: 1450 g TST: 360 min TIT: 460 min WIT: 35 min BBP: None |
Tacrolimus, mycophenolate and intraoperative corticoid bolus and tampering. Received PMT for acute cellular rejection treatment |
11st POD Subfebrile temperature dry cough |
Supportive care Discharged on 27th POD |
| 5 |
34 y, male BMI: 22.38 kg/m2 |
Sclerosing primary cholangitis MELD: 35 Child B7 |
None |
Female, 42 y BD: ischemic stroke |
GW: 1425 g TST: 395 min TIT: 360 min WIT: 35 min BBP: None |
Tacrolimus and intraoperative corticoids bolus with tampering. Mycophenolate, PMT, anti‐thymocyte globulin were later used due to steroid‐resistant severe acute cellular rejection |
18th POD: asymptomatic 24th POD: Fever Mild dyspnea |
Supportive care Needle thoracocentesis drainage of pleural effusion Discharged home on 41st POD |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BBP, blood‐borne products; BD, brain death; BMI, body mass index; DM, diabetes mellitus; GGT, gamma‐glutamyl transferase; GW, graft weight; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; ICU, intensive care unit; MELD, model of end‐stage liver disease; NASH, non‐alcoholic steatohepatitis; PMT, pulse methylprednisolone therapy POD, postoperative day; SHA, systemic artery hypertension; TIT, total ischemic time; TST, total surgery time; WIT, warm ischemic time.
Table 2.
Laboratory assessment on COVID‐19 diagnosis
| Laboratory test | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Alkaline phosphatase (U/L) | 571 | 398 | 103 | 646 | 906 |
| gamma‐glutamyl transferase (U/L) | 1288 | 1405 | 283 | 3211 | 2167 |
| Total Bilirubin (mg/dL) | 0.74 | 1.39 | 0.68 | 4.51 | 2.73 |
| Direct Bilirubin (md/dL) | 0.50 | 1.2 | 0.46 | 4.03 | 2.45 |
| Aspartate aminotransferase (U/L) | 251 | 121 | 53 | 116 | 43 |
| Alanine aminotransferase (U/L) | 150 | 137 | 106 | 209 | 65 |
| Albumin (g/dL) | 1.8 | 2.2 | 3.3 | 2.5 | 2.6 |
| INR | — | 1.1 | 1.07 | 1.06 | 1.10 |
| Total leukocytes (/mm3) | 29 340 | 12 910 | 3130 | 24 850 | 14 740 |
| Lymphocyte count (/mm3) and percentage | 1174 (4%) | 387 (2.99%) | 330 (10.54%) | 1740 (7%) | 737 (5%) |
| Creatinine (mg/dL) a | 5.84 | 6.36 | 1.0 | 3.04 | 1.07 |
| Urea (mg/dL) a | 178 | 141 | 36 | 50 | 18 |
| C‐reative protein (mg/L) | 146.12 | 226.01 | 96.3 | 50.50 | 82.22 |
| D‐dimer (ug/mL) | 8477 | 19 354 | 4626 | 2253 | 6489 |
| Lactate dehydrogenase (U/L) | 895 | 691 | 280 | — | — |
| Ferritin (ng/mL) | — | 2896 | — | — | 1558 |
| Tacrolimus serum level (ng/mL) | 10.8 | 13.1 | 13.3 | 13.4 | 13.7 |
Patients 1, 2, and 4 required renal replacement therapy.
2.1. Case 1
A 69 years old female patient was presented with hepatitis C virus infection DDLT due to cirrhosis and hepatocellular carcinoma (HCC), which were downstaged to within Milan criteria after 4 transarterial chemoembolization (TACE). Her past medical history was remarkable for systemic arterial hypertension, coronary disease, and pulmonary hypertension. Myocardial perfusion scintigraphy did not show any inactive areas and ejection fraction was 69% on echocardiogram, even though coronary angiography identified important stenosis (>80%) in the right posterior descending artery, descending anterior artery and circumflex artery.
The procedure went on uneventfully, and the patient was extubated on the 1st postoperative day (POD). Renal function was worsened, and dialysis was required from the 2nd POD. She presented with mild dyspnea, fever, and diarrhea on 9th POD. A thoracic computed tomography (CT) scan showed multiple bilateral ground‐glass pulmonary opacities, occasionally associated with interlobular septa thickening and fine reticulate, affecting approximately 50% of the lung. A RT‐PCR in NO swab confirmed SARS‐CoV‐2 infection, and she was started on azithromycin. The patient clinically worsened on 12th POD, presenting hypotension and massive dyspnea, and had to undergo orotracheal intubation (OTI). Despite immunosuppression discontinuation, she developed important hemodynamic instability and died on 13th POD due to refractory shock and acidosis.
2.2. Case 2
A 67 years old male patient underwent DDLT 10 days before the COVID‐19 quarantine was declared. He was a cirrhotic patient due to non‐alcoholic steatohepatitis (NASH) and α1‐antitripsin deficiency, who had additional points in waitlist because of hepatic encephalopathy. The postoperative course was remarkable for persistent need of hemodialysis. On 26th PO, liver enzymes were mildly elevated and he underwent a percutaneous liver biopsy, which did not show significant alterations, but cytomegalovirus infection was diagnosed (serum polymerase chain reaction: 213 458 IU/mL) and he was started on intravenous ganciclovir. On 36th POD, he was presented with fever, hypoactive delirium, and progressive dyspnea. Thoracic CT scan showed bilateral several ground‐glass pulmonary opacities affecting approximately 50% of the lungs (Figure 1A,B). He underwent OTI due to persistent hypoxemia and respiratory discomfort. A RT‐PCR in tracheal secretion was positive for SARS‐CoV‐2. He was then started on large‐spectrum antibiotics (meropenem and vancomycin) for 14 days as well as oseltamivir, azithromycin, and hydroxychloroquine for 5 days. Mycophenolate was withdrawn, and tacrolimus serum level was maintained at around 5 mg/dL.
Figure 1.
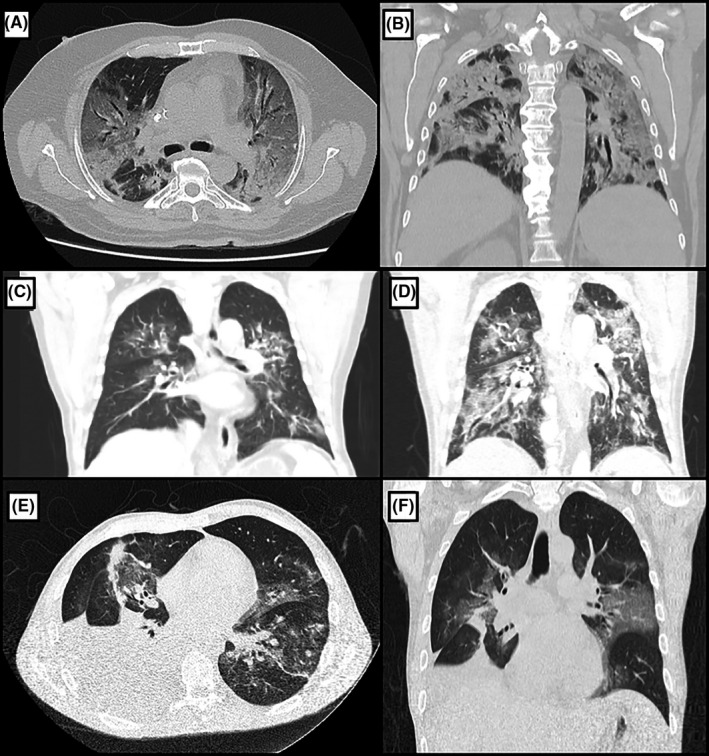
A and B, Thoracic computed tomography (CT) scan of patient 2, showing bilateral several ground‐glass pulmonary opacities affecting approximately 50% of the lungs, occasionally associated with thickening of interlobular septa and thin reticulate, in addition to peripheral sparse consolidation foci with greater extension in the posterior aspect of the lower lobes (A—axial view, B—coronal view). C, Thoracic CT scan of patient 3 on 10th postoperative day (POD), showing bilateral multiple ground‐glass pulmonary opacities, sometimes associated with thickening of interlobular septa and fine reticulate, affecting less than 50% of the lungs. D, Thoracic CT scan of the same patient on 20th POD, performed due to shortness of breath worsening, revealing increase in number and dimensions of ground‐glass pulmonary opacities, now affecting more than 50% of the lungs. E and F, Thoracic CT scan of patient 5, showing numerous bilateral peribronchovascular ground‐glass opacities, mainly in the upper lobes, some with thickening of the inter and intralobular septa. There is also a large pleural effusion on the right side with restrictive atelectasis of the adjacent pulmonary parenchyma (E—axial view, F—coronal view)
He remained on mechanical ventilation for 20 days. Ventilator‐associated pneumonia was diagnosed on 53th POD with multiresistant Acinetobacter baumannii being identified on tracheal secretion, and he was started on colistin. His condition continued to deteriorate and he died of persistent hypoxemia and hemodynamic instability on 56th POD.
2.3. Case 3
A 69 years old male patient underwent DDLT due to alcoholic cirrhosis and HCC. Postoperative course was uneventful, and he was discharged home on 8th POD. He returned to the emergency department on 10th POD complaining of fever, watery diarrhea, dry cough, and mild exertional dyspnea. A thoracic CT scan showed bilateral multiple ground‐glass pulmonary opacities (Figure 1C), affecting less than 50% of the lungs. He was started on oseltamivir for 5 days, piperacillin/ tazobactam for 7 days, and oral metronidazole for 10 days. An RT‐PCR in NO swab was positive for SARS‐CoV‐2. Shortness of breath worsened on the 10th day of hospitalization. Another thoracic CT scan showed increase in number and dimensions of ground‐glass opacities, now affecting more than 50% of the lungs (Figure 1D). He could nonetheless sustain adequate levels of O2 saturation on Venture mask 50% and did not require further interventions, being discharged home on 17th hospitalization day.
2.4. Case 4
A 59 years old male patient with cryptogenic cirrhosis underwent an uneventful DDLT. The patient was extubated on 1st POD, but required hemodialysis from the 4th POD. Pulse methylprednisolone therapy (PMT) was used on 6th POD due to ACR. On 11st POD, he developed subfebrile temperature and dry cough, without need of oxygen therapy. A thoracic CT scan showed bilateral ground‐glass pulmonary opacities, sometimes associated with thickening of interlobular septa and fine reticulate. An RT‐PCR in NO swab confirmed SARS‐CoV‐2. Meropenen and Vancomycin were used for 3 days, and mycophenolate was withdrawn. The patient presented satisfactory evolution and did not require further interventions, being discharged on 27th POD.
2.5. Case 5
A 34 years old male patient with sclerosing primary cholangitis and cirrhosis underwent DDLT with biliodigestive anastomosis. Postoperative evolution was complicated by severe ACR, which did not respond to PMT. He underwent percutaneous liver biopsy, which complicated with hemorrhage and required exploratory laparotomy to evacuate blood clots on 11st POD. Owing to the diagnosis of steroid‐resistant severe ACR, mycophenolate and anti‐thymocyte globulin were introduced. On 18th POD, an abdominal CT scan performed to evaluate the presence of retained clots showed some ground‐glass opacities in the base of the left lung. He started with fever and mild dyspnea on 24th POD and RT‐PCR in NO swab confirmed SARS‐CoV‐2. A thoracic CT scan showed numerous bilateral peribronchovascular ground‐glass opacities, mainly in the upper lobes. Almost total atelectasis of the right lower lobe due to adjacent pleural effusion was also noted, which was eventually drained via needle thoracocentesis (Figure 1E,F). Despite the more aggressive immunosuppression, the patient remained well with adequate O2 saturation on oxygen catheter and was discharged home on 41st POD.
3. DISCUSSION
This report represents one of the largest series of liver transplant recipients in early postoperative with nosocomial COVID‐19. Fever and respiratory symptoms were present in all cases, and gastrointestinal manifestations were observed in two. We could identify two patterns of clinical evolution: some patients presented mild disease while others required mechanical ventilation and eventually died. As appointed for post‐transplant patients with long‐term metabolic complications, 3 comorbidities seem to outweigh immunosuppression in determining prognosis even in the early postoperative period. In fact, one patient with unfavorable outcome had severe coronary disease and the other one experienced prolonged postoperative course complicated with CMV infection and kidney failure. Both were also obese. Whereas the first patient developed SARS‐CoV‐2 infection and rapidly progressed to death, the second exhibited a more insidious course and ultimately died of secondary bacterial infection. Regarding laboratory assessment, a wide variation in liver enzymes was observed, probably related to the particular evolution of each case before COVID‐19 diagnosis. However, inflammatory markers and D‐dimer were elevated in all cases, even though they are not specific for COVID‐19.
Regarding the specify treatment of COVID‐19, we followed our institution's protocol, in which most novel drugs, such as umifenovir, lopinavir/ritonavir, and tocilizumab, 2 , 4 , 9 , 12 , 16 are used under clinical trials. Patients 1 and 2 developed severe COVID‐19 and required OTI. They were started on azithromycin, but hydroxychloroquine was used only in the second case, because the first one rapidly progressed to death. Hydroxychloroquine was initially recommended, but is no longer used given the latest evidence. Large‐spectrum antibiotics were used in patients 3 and 4 aiming to cover bacterial infection as well; however, as they presented satisfactory clinical evolution, no additional treatment was performed. Patient 5 experienced mild symptoms and successfully responded to supportive care.
Immunosuppression was completely withdrawn in case 1, due to the patient's dramatic worsening. In case 2, mycophenolate was discontinued and tacrolimus serum levels were lowered. In case 4, mycophenolate was also discontinued, but no changes were made in tacrolimus serum levels. In cases 3 and 5, immunosuppression was not altered. The risk of ACR is higher in early transplanted patients and, in fact, it occurred in 2 early cases previously described in literature. 7 , 13 It seems that immunosuppression must be tailored on a case‐by‐case basis according to the clinical evolution. For instance, patient 4 underwent PMT before COVID‐19 diagnosis due to ACR and patient 5 underwent PMT and anti‐thymocyte globulin infusion owing to steroid‐resistant ACR. Despite being severely immunosuppressed, they presented only mild symptoms.
As a public quaternary center, our institution was prepared to be the main reference center of COVID‐19 cases in São Paulo State at the beginning of April, 2020. As the number of transplants in the context of COVID‐19 pandemic has decreased substantially, 16 , 18 , 19 strategies have been developed for ensuring the availability of transplantation activity, such as establishment of hospital areas especially dedicated to non‐COVID‐19 patients. 20 , 21 The main building of our institution was then designated exclusively for treating patients with COVID‐19 when the quarantine was declared, while the nearby institutes were considered areas with low exposure to SARS‐CoV‐2. Organ transplantation activities were supposed to continue in wards and ICUs without SARS‐CoV‐2‐infected patients. Furthermore, all deceased donors were screened to SARS‐CoV‐2 via RT‐PCR in NO swab and all recipients performed a thoracic CT scan before hospital admission, in accordance with Brazilian Association of Organ Transplantation (ABTO) recommendations, except patient 2 who was transplanted immediately before the implementation of these measures. All donors tested negative for SARS‐CoV‐2, and no alterations were noted on recipients CT scans.
The incubation period for SARS‐CoV‐2 is usually 5‐6 days; however, it may reach 14 days. 21 Therefore, it is possible that 3 patients (cases 1, 3, and 4) might have been admitted to the hospital already infected. We consider this hypothesis unlikely, since all of them were clinically asymptomatic and with no radiological sign, and there was an ongoing nosocomial outbreak of COVID‐19 in our institution at that time, with many non‐transplant patients from others non‐COVID‐19 wards developing the disease. Therefore, it is reasonably safe to assume that we experienced a nosocomial spread of the virus, probably carried out by asymptomatic patients, or even by patient's visitors and healthcare personnel. Due to these events, infection control protocols were tightened, including a temporary ban on family visits, strengthening of barriers precautions, and mass SARS‐CoV‐2 screening for healthcare professionals. We also included the RT‐PCR for SARS‐CoV‐2 in NO swab in the recipients screening. Although those infection control protocols with strict barrier precautions may reduce SARS‐CoV‐2 nosocomial spread even in immunosuppressed patients, 22 the challenge of maintaining large liver transplant programs in areas with high community transmission of SARS‐CoV‐2 will remain until a vaccine is developed.
In conclusion, we reported a case series of liver recipients who developed SARS‐CoV‐2 infection in the early postoperative period, probably related to a nosocomial outbreak. Our experience highlights that COVID‐19 may impact negatively the postoperative course, especially in elder and obese patients with comorbidities, and draws attention to COVID‐19 nosocomial spread.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest with regards to the content of this manuscript.
AUTHOR CONTRIBUTIONS
DRW, EA, and LSN involved in conceptualization and wrote the original draft. VR, LNG, RBM, RSP, and RMA investigated the study. CSL and LMM involved in resources. LBH, LD, and DRT involved in data curation. FHG and WA wrote, reviewed, and edited the study. LAC involved in supervision.
Waisberg DR, Abdala E, Nacif LS, et al. Liver transplant recipients infected with SARS-CoV-2 in the early postoperative period: Lessons from a single center in the epicenter of the pandemic. Transpl Infect Dis. 2021;23:e13418. 10.1111/tid.13418
REFERENCES
- 1. Serdan TDA, Masi LN, Gorjao R, Pithon‐Curi TC, Curi R, Hirabara SM. COVID‐19 in Brazil: historical cases, disease milestones, and estimated outbreak peak . Travel Med Infect Dis. 2020:e101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu B, Wang Y, Zhao Y, Shi H, Zeng F, Chen Z. Successful treatment of severe COVID‐19 pneumonia in a liver transplant recipient. Am J Transplant. 2020;20(7):1891‐1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID‐19 in long‐term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5(6):532‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang JF, Zheng KI, George J, et al. Fatal outcome in a liver transplant recipient with COVID‐19. Am J Transplant. 2020;20(7):1907‐1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Webb GJ, Moon AM, Barnes E, Barritt AS, Marjot T. Determining risk factors for mortality in liver transplant patients with COVID‐19. Lancet Gastroenterol Hepatol. 2020;5(7):643‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020;20(7):1849‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lagana SM, De Michele S, Lee MJ, et al. COVID‐19 associated hepatitis complicating recent living donor liver transplantation. Arch Pathol Lab Med. 2020. [DOI] [PubMed] [Google Scholar]
- 8. Kates OS, Fisher CE, Stankiewicz‐Karita HC, et al. Earliest cases of coronavirus disease 2019 (COVID‐19) identified in solid organtransplant recipients in the United States. Am Transplant. 2020;20(7):1885‐1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pereira MR, Mohan S, Cohen D, et al. COVID‐19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maggi U, De Carlis L, Yiu D, et al. The impact of the COVID‐19 outbreak on liver transplantation programmes in Northern Italy. Am J Transplant. 2020;20(7):1840‐1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lauterio A, De Carlis R, Belli L, Fumagalli R, De Carlis L. How to guarantee liver transplantation in the north of Italy during the COVID‐19 pandemic. A sound transplant protection strategy. Transpl Int. 2020. [DOI] [PubMed] [Google Scholar]
- 12. Hammami MB, Garibaldi B, Shah P, et al. Clinical course of COVID‐19 in liver transplant recipient on hemodialysis and response to tocilizumab therapy: a case report. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhong Z, Zhang Q, Xia H, et al. Clinical characteristics and immunosuppressant management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20(7):1916‐1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qin J, Wang H, Qin X, et al. Perioperative presentation of COVID‐19 disease in a liver transplant recipient. Hepatology. 2020. [DOI] [PubMed] [Google Scholar]
- 15. Lee BT, Perumalswami PV, Im GY, Florman S, Schiano TD. COVID‐19 in liver transplant recipients: an initial experience from the U.S. Epicenter. Gastroenterology. 2020;S0016‐S5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nacif LS, Zanini LY, Waisberg DR, et al. COVID‐19 in solid organ transplantation patients: a systematic review. Clinics (Sao Paulo). 2020;75:e1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(3):e2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vatche Agopian V, Verna E, Goldberg D. Changes in liver transplant center practice in response to COVID‐19: unmasking dramatic center‐level variability. Liver Transpl. 2020.26(8):1052–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boyarsky BJ, Po‐Yu Chiang T, Werbel WA, et al. Early impact of COVID‐19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20(7):1809‐1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lauterio A, De Carlis R, Belli L, Fumagalli R, De Carlis L. How to guarantee liver transplantation in the north of Italy during the COVID‐19 pandemic: a sound transplant protection strategy. Transplant Int. 2020;33(8):969–970. [DOI] [PubMed] [Google Scholar]
- 21. Sandes‐Freitas TV, Canito Brasil IR, Oliveira Sales MLMB, et al. Lessons from SARS‐CoV‐2 screening in a Brazilian organ transplant unit. Transplant Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamar N, Esposito L, Hebral AL, Guitard J, Del Bello A. Specific organization for in‐hospital belatacept infusion to avoid nosocomial transmission during the SARS‐CoV‐2 pandemic. Am J Transplant. 2020;20(10):2962–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]


