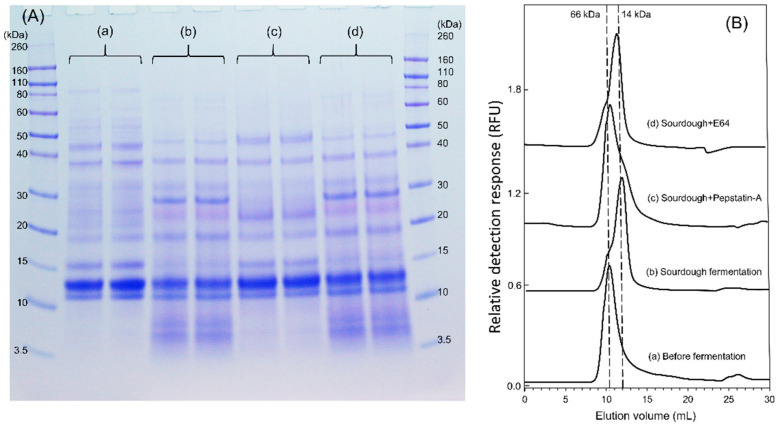
Figure 2.
Effect of protease inhibitors on the wheat sourdough albumin/globulin extract and fluorescence-labelled ATI tetramer. (A) SDS-PAGE of albumin/globulin extract. (a) control before fermentation, (b) rye starter-wheat sourdough, pH was 3.6 after 17 h fermentation, (c) rye starter-wheat sourdough in the presence of 20 µM aspartic proteinase inhibitor Pepstatin-A, pH was 3.7 after 17 h fermentation, (d) rye starter-wheat sourdough in the presence of 10 µM cysteine proteinase inhibitor E-64, pH was 3.6 after 17 h fermentation. The replicates were from two individual fermentation. (B) Size-exclusion chromatogram of fluorescein isothiocyanate (FITC)-labelled ATI tetramer after sourdough fermentation in the presence of protease inhibitors. The samples were treated the same as in (A). Fluorescent detector was set at excitation 488 nm and emission 530 nm. Elution volume of molecular marker bovine serum albumin (66 kDa) and lysozyme (14 kDa) was indicated in broken lines.