Abstract
Fine-needle aspiration (FNA) is among the first diagnostic tools used in the evaluation of thyroid nodules. It has the ability to triage patients with benign and malignant lesions, thus defining the optimum clinical and/or surgical management. The Bethesda System for Reporting Thyroid Cytopathology has found worldwide acceptance. Thyroid FNA offers high positive predictive value (97%–99%), with sensitivities and specificities of 65% to 99% and 72% to 100%, respectively. Nonetheless, many potential diagnostic pitfalls exist that can lead to false-positive and/or false-negative results. This article discusses several of the potential pitfalls in the cytologic evaluation of thyroid lesions.
Keywords: Fine-needle aspiration, Cytology, Thyroid nodule, Thyroid cancer, False-positive diagnoses, False-negative diagnoses
OVERVIEW
Thyroid nodules are frequently detected in clinical practice, reflecting their prevalence in the general population and especially among women.1,2 Fine-needle aspiration (FNA) is among the first and most valuable diagnostic tools for the presurgical discrimination of benign and malignant lesions. Although most thyroid nodules (90%–92%) are benign, the small subset of malignant nodules needs to be presurgically identified for optimal management.3–6 The diagnostic value and accuracy of FNA in the evaluation of thyroid nodules has been well established in the literature.1–8 Thyroid FNA has shown a high positive predictive value for identifying malignancy, ranging from 97% to 99% in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) to 95% from the Royal College of Pathologists in the British thyroid system.1,9 Using TBSRTC, Yang and colleagues10 reported a sensitivity of 94% and specificity of 98.5% for malignancy, and sensitivity of 89.3% and specificity of 74% for identifying neoplastic disease.
Despite its overall success, FNA false-positive (FP) and/or false-negative (FN) results can occur for a variety of reasons, often caused by the quality of the aspirated material (Table 1). Yang and colleagues10 found an overall 15.3% discrepancy rate between cytologic and histologic diagnoses, with FN results related mostly to issues of sample adequacy. Overall, approximately 3.2% of patients with a benign FNA result have been shown to be FNs. However, most studies only considered the FNA results of patients who proceeded to surgery.10–21 FP results of thyroid FNA have a broad range of causes, but many are caused by tumors that can show a range of cytomorphologic appearances that overlap with other tumor subtypes. This overlap is seen in FNA of tumors such as medullary thyroid carcinoma (MTC), anaplastic thyroid carcinoma (ATC), and in certain variants of papillary thyroid carcinoma (PTC). This article discusses several of the important diagnostic pitfalls for a range of diagnostic categories and tumor types that can be encountered in thyroid cytology and that can lead to both FN and FP thyroid FNA results.
Table 1.
Examples of false-negative and false-positive thyroid fine-needle aspiration results
| Diagnoses | FN | FP |
|---|---|---|
| ND | Few cells suggestive of benign condition | AUS/FLUS |
| Cystic lesions | Misinterpretation of cystic degeneration and squamous cells | Atypical cyst-lining cells, few cells with features suggestive for SFM or even PM |
| GD | None | PTC |
| LT | Scant diagnostic features of LT | Mostly PTC; lymphomas; FNHCT |
| Follicular-patterned lesions | Underestimate architectural and cellular features; intrathyroidal parathyroid adenoma; PTEN hamartoma | SFM/PM; PTC; FVPTC; FC |
| Hürthlecell neoplasm | HT; goiter, granular cell tumor; intrathyroidal parathyroid adenoma | PTCa; MTC; HTC |
| NIFTP | Follicular-patterned lesions | SFM or PM favoring PTC; MTC |
| PTC | HTT; LT; GD | MTC; PDC |
| FVPTC | AUS/FLUS or SFN/FN | NIFTP |
| NIFTP | AUS/FLUS; BL | SFM/PM |
| MTC | SFN/FN; FNHCT | PTC, HTC |
| PDC | SFN/FN; FNHCT | MTC; metastases; lymphoproliferative disorders |
| ATC | Few atypical cells classified as ND | PDC; MTC; metastases; lymphomas |
| Metastatic lesions | Few atypical cells classified as ND | PDC, MTC, ATC |
Abbreviations: ATC, anaplastic thyroid carcinoma; AUS, atypia of undetermined significance; FC, follicular carcinoma; FLUS, follicular lesion of undetermined significance; FNHCT, follicular neoplasm, Hürthle cell type; FVPTC, follicular variant of PTC; GD, Graves disease; HT, Hashimoto thyroiditis; HTC, Hürthle cell carcinoma; LT, lymphocytic thyroiditis; MTC, medullary thyroid carcinoma; ND, nondiagnostic; NIFTP, noninvasive follicular neoplasm with papillarylike nuclear features; PDC, poorly differentiated carcinoma; PM, positive for malignancy; PTC, papillary thyroid carcinoma; PTEN, phosphatase and tensin homolog; SFM, suspicious for malignancy; SFN/FN, suspicious for follicular neoplasm/follicular neoplasm.
Including oncocytic variant of PTC.
NONDIAGNOSTIC THYROID FINE-NEEDLE ASPIRATIONS
Thyroid specimens that are inadequate for cytologic interpretation are classified as nondiagnostic (ND) in TBSRTC.1 Different factors can contribute to an ND result, such as the inherent nature of the nodule (cystic vs solid vs fibrotic and calcified), as well as aspects of the FNA procedure and operator experience. In order to reduce the rate of ND results, the recommendation for these samples is to repeat the FNA under sonographic guidance targeting cellular areas of the nodule. Using this approach, several studies have documented a significant reduction (from 70% to 83%) in the ND rate.14,22–25 TBSRTC reports a risk of malignancy (ROM) of 5% to 10% for ND samples.1 PTC has been reported as the most frequent cause of an FN diagnosis in the ND category.22–25 One important caveat in the evaluation of a thyroid FNA that might otherwise be interpreted as ND is that the presence of cytologic atypia should be reported as atypia of undetermined significance(AUS) or follicular lesion of undetermined significance (FLUS) even without the required minimal number of follicular cells.1
CYSTIC THYROID LESIONS
Cystic or predominantly cystic thyroid nodules are reported in approximately 15% to 25% of thyroid lesions and most are histologically benign.22–26 The microscopic findings include foamy and hemosiderin-laden macrophages, scant colloid, and few follicular cells. On occasion, cyst-lining cells can show atypical nuclear features that can mimic PTC (Fig. 1). FNA of cystic thyroid nodules has been associated with both FN and FP results.
Fig. 1.

FNA of cystic thyroid nodule. Rare groups of follicular cells with nuclear atypia are present. These atypical cyst-lining cells can mimic PTC (Papanicolaou stain, original magnification ×600).
The ROM is generally low for thyroid nodules, with low suspicion ultrasonography pattern that is less than 2–3 cm in size.26 The ROM, particularly for PTC, increases with larger and more complex cysts.1 Jaragh and colleagues27 analyzed 76 cyst fluid-only FNA cases, concluding that the only morphologic feature predictive of malignancy was the presence of follicular epithelium with atypical features. However, some atypical cells in benign cystic lesions can be derived from cyst-lining cells as epithelial repair.28,29 Specific cytomorphologic features are associated with cyst-lining cells including (1) well-defined cellular borders, (2) dense granular cytoplasm, (3) enlarged nuclei with regular nuclear borders, and (4) occasional pale nuclei. A diagnosis of AUS/FLUS is considered more appropriate for these cases.1
In rare instances, squamous cells can be encountered in cystic thyroid FNAs, where they can be a diagnostic pitfall.28–33 Their presence has been associated with a variety of different entities, including benign lymphoepithelial cysts, epidermoid cyst, thyroglossal duct remnants, and squamous metaplasia in long-standing Hashimoto thyroiditis (HT). Much less often, squamous cells reflect a malignant lesion such as PTC, ATC, primary or metastatic squamous carcinoma, mucoepidermoid carcinoma, or carcinoma with thymuslike differentiation. Gage and colleagues28 analyzed a series of 15 thyroid lesions with a predominance of squamous cells, and suggested the presence of 3 main cytologic patterns: benign, mixed cellularity, and malignant. Most commonly, detection of abundant anucleated and bland squamous cells plus background lymphocytes was compatible with a benign lesion. In some cases, the squamous cells represented reactive squamous metaplasia in HT, whereas malignant lesions were usually easily recognized.32
GRAVES DISEASE
Graves disease (GD) is a common endocrine disorder that can pose diagnostic issues because of cellular changes overlapping with the nuclear features of PTC.1,34,35
GD is among the most frequent causes of hyperthyroidism, with a prevalence of palpable thyroid nodules 3-fold higher than in the general population, and FNA of these nodules can represent a significant diagnostic dilemma. Despite the low ROM (between 1.9% and 2.5%), the cytomorphologic changes in GD, especially after treatment, can be misinterpreted as PTC.34,35 Cytologic features found in aspiration of GD include scattered follicular cell pleomorphism, fire-flare cells, Hürthle cell changes, and background lymphocytes. Because of the nonspecific nature of these findings, clinical correlation is essential to avoid an FP diagnosis. Treatment of GD with radioactive iodine is well known to cause significant follicular cell atypia, including nuclear and cellular enlargement, anisonucleosis, coarse chromatin, hyperchromasia, and cytoplasmic vacuolization. To avoid an FP diagnosis, it is helpful to note that these samples lack the fine powdery chromatin typically seen in PTC. In the attempt to differentiate GD from PTC, Anderson and colleagues34 evaluated 11 cytomorphologic features in FNA cases of GD and PTC in GD. They concluded that only 4 of these features were predictive of PTC in GD, and all were based on nuclear characteristics, including oval nuclear shape, pale chromatin, and distinct eccentric nucleoli.32
INFLAMMATORY LESIONS
LYMPHOCYTIC THYROIDITIS
The term lymphocytic thyroiditis (LT) encompasses a variety of conditions ranging from chronic lymphocytic thyroiditis (HT) to subacute lymphocytic thyroiditis (postpartum and silent thyroiditis) and focal (silent) thyroiditis.1 Other entities with LT include GD, nodular goiter, and immunoglobulin G4 thyroiditis.1 HT represents both the most common form of autoimmune thyroiditis and the most common cause of hypothyroidism. It typically affects middle-aged women as a diffuse heterogeneous enlargement of the thyroid, but frequently the enlargement is localized as a pseudonodule (from 26% to 80% of cases) and raises suspicion of a neoplastic nodule.36–39 HT is recognized as a multifaceted disease with an initial phase of hyperthyroidism followed by a chronic phase of hypothyroidism. In the initial phase of HT, there is antibody-mediated destruction of follicular structures with lymphocytic infiltration. The chronic phase shows the presence of atrophic follicles and fibrotic parenchyma. Depending on the stage of the disease, FNA specimens can result in a range of diagnostic pitfalls.37–39 For instance, the prevalence of either the lymphoid or the oncocytic component may raise the possibility of lymphoma or a Hürthle cell neoplasm, respectively. The early phase of hyperthyroidism is characterized by a population of oncocytic cells arranged in flat sheets or as isolated cells. In some cases, the oncocytic cells show nuclear enlargement, grooves, and chromatin clearing, suggesting PTC (Fig. 2). FNA samples with a prominent population of lymphoid cells where lymphoma is a concern can be evaluated by flow cytometry.38,39
Fig. 2.
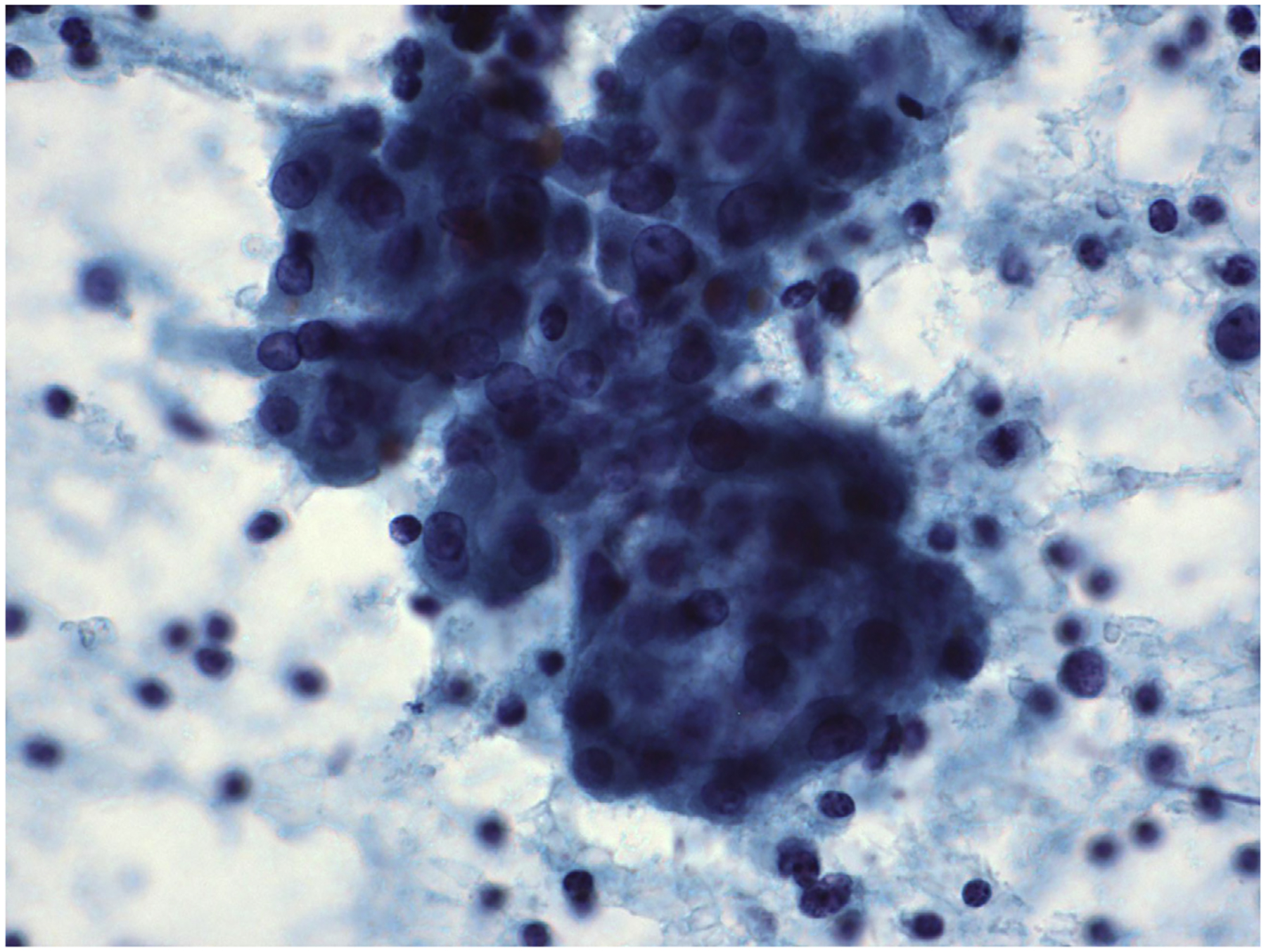
FNA of chronic LT. The follicular cells are cohesive and have moderate amounts of oncocytic cytoplasm and mild nuclearatypia (Papanicolaou stain, original magnification ×600).
A recent study by Yi and colleagues40 showed a higher prevalence of thyroiditis among patients with FP results than among those with PTC. Among 48 patients with FP results, thyroiditis was diagnosed in 54.2% (26 cases), whereas the rate of thyroiditis was only 9.4% in patients with PTC. A key point is that, in the presence of a lymphocytic background, a definitive diagnosis of PTC should be made only when well-developed classic features of PTC are present.
A unique entity in patients with HT is the Warthin-like variant of PTC, which mimics the mixture of oncocytes and lymphocytes of HT1 (Fig. 3). However, a careful evaluation of the cytologic features can resolve the dilemma. The Warthin-like variant of PTC has more pleomorphic and irregular nuclear membranes, nuclear pseudoinclusions, and less prominent nucleoli than the oncocytes of HT. In addition, the Warthin-like variant of PTC shows permeating lymphocytes and plasma cells.
Fig. 3.
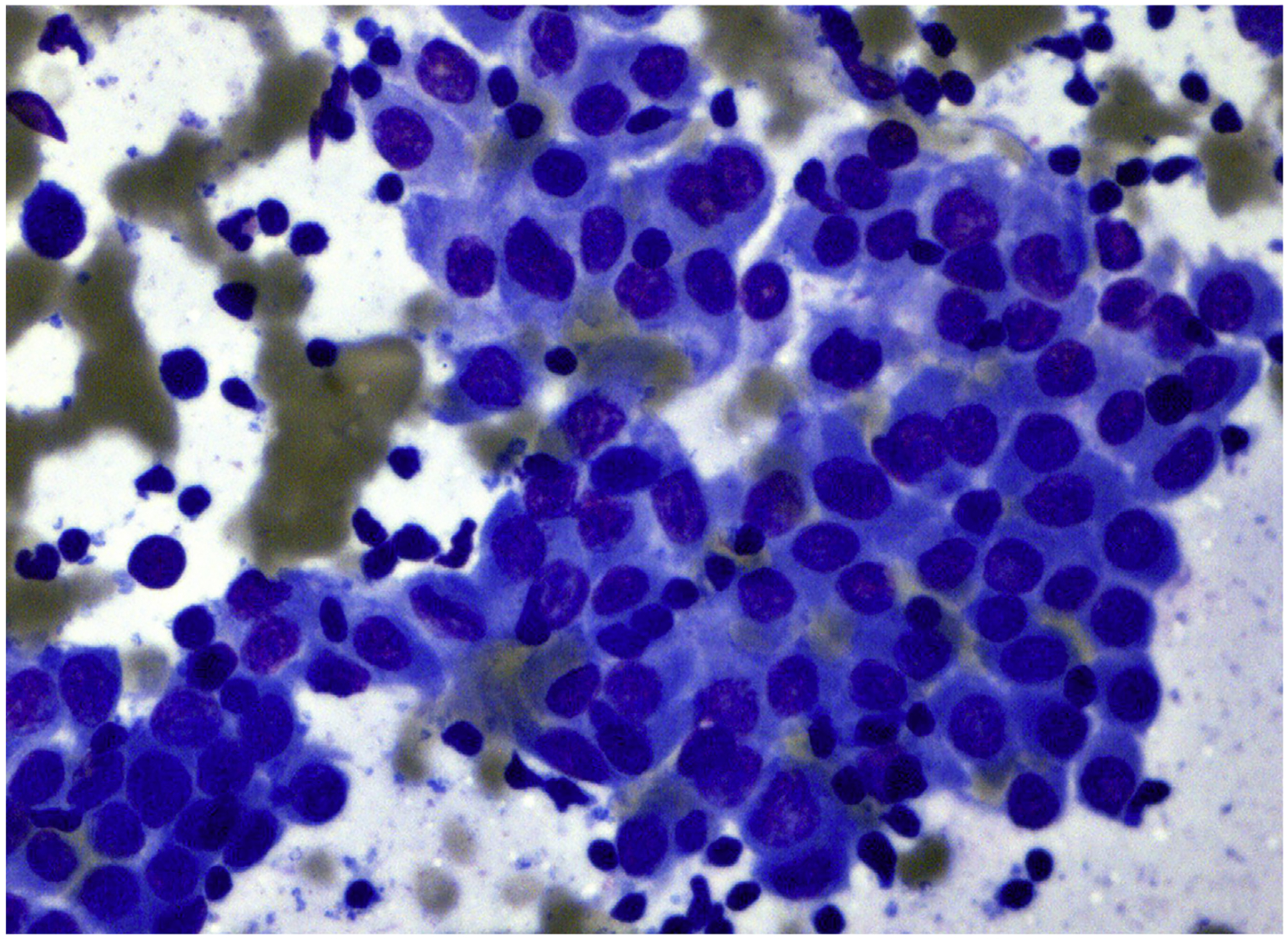
FNA of Warthin-like variant of PTC. Cells have oncocytic features and nuclear atypia in a background of chronic inflammation (Diff-Quik stain, original magnification ×600).
GRANULOMATOUS THYROIDITIS
Granulomatous (de Quervain) thyroiditis (GT) is a self-limiting inflammatory process of the thyroid that is usually diagnosed clinically in patients with neck and ear pain and tenderness occurring a few weeks after a viral upper respiratory tract infection.40–45 In rare cases, FNA is performed for nodular swelling that raises the possibility of a neoplastic condition. The cytologic features are variable and depend on the stage of disease. The initial stage shows neutrophils and eosinophils resembling an acute thyroiditis; the later stages show hypocellularity with giant cells, epithelioid cells, lymphocytes, macrophages, and scant degenerated follicular cells. In the involutional stage, there might only be giant cells and inflammatory cells. In the presence of granulomas, other entities, including sarcoidosis and infections, should be considered. Solano and colleagues42 retrospectively analyzed 36 cases of GT to reassess the clinical and cytologic findings characteristic of GT: presence of follicular cells with intravacuolar granules and/or plump transformed follicular cells; epithelioid granulomas; multinucleated giant cells; an acute and chronic inflammatory background; absence of fire-flare cells, hypertrophic follicular cells, oncocytic cells, and transformed lymphocytes. Based on these findings, selected features of GT can mimic a variety of entities, such as hemorrhage and infarction in a nodular goiter, and final stages of HT.
INDETERMINATE FOLLICULAR-PATTERNED LESIONS
ATYPIA OF UNDETERMINED SIGNIFICANCE/FOLLICULAR LESION OF UNDETERMINED SIGNIFICANCE AND FOLLICULAR NEOPLASM/SUSPICIOUS FOR FOLLICULAR NEOPLASM
One of the most important challenges in thyroid cytology is represented by the so-called gray zone of indeterminate thyroid FNA lesions. Despite most thyroid lesions being correctly classified as either benign (70%–75%) or malignant (5%–10%), the remaining nodules (20%–25%) belong to indeterminate categories.46–55 The interpretation of follicular-patterned lesions is challenging and difficulties and limitations in discriminating whether these nodules are benign or malignant entities can result in unnecessary surgical resections (lobectomy or total thyroidectomy) and increased health care costs.46,54–65
The atypia in AUS/FLUS includes a range of nuclear and/or architectural changes.1,66,67 Both nuclear and architectural patterns can result in diagnostic pitfalls even though most of these findings are caused by benign conditions such as hyperplastic-adenomatous nodules, toxic adenomas, and chronic LT. Two benign instances in which AUS/FLUS diagnosis can be avoided are the presence of few oncocytic cells and/or cyst-lining cells with mild atypia mixed with benign follicular cells, and the presence of papillary structures without any nuclear features of PTC.
Intrathyroidal parathyroid adenomas are often misclassified as suspicious for follicular neoplasm (SFN). Such aspirates can show a microfollicular pattern.68–70 The ultrasonography detection of a posterior nodule, small hyperchromatic nuclei, scant cytoplasm, and crowded trabecular clusters without colloid can be a subtle clue for the diagnosis. The application of ancillary techniques in such instances, including immunostains for parathyroid hormone (PTH) and/or molecular analysis by Afirma or Thyroseqv 3, can recognize the expression profile supporting the diagnosis of a PTH lesion11,17,71–77 (Fig. 4).
Fig. 4.
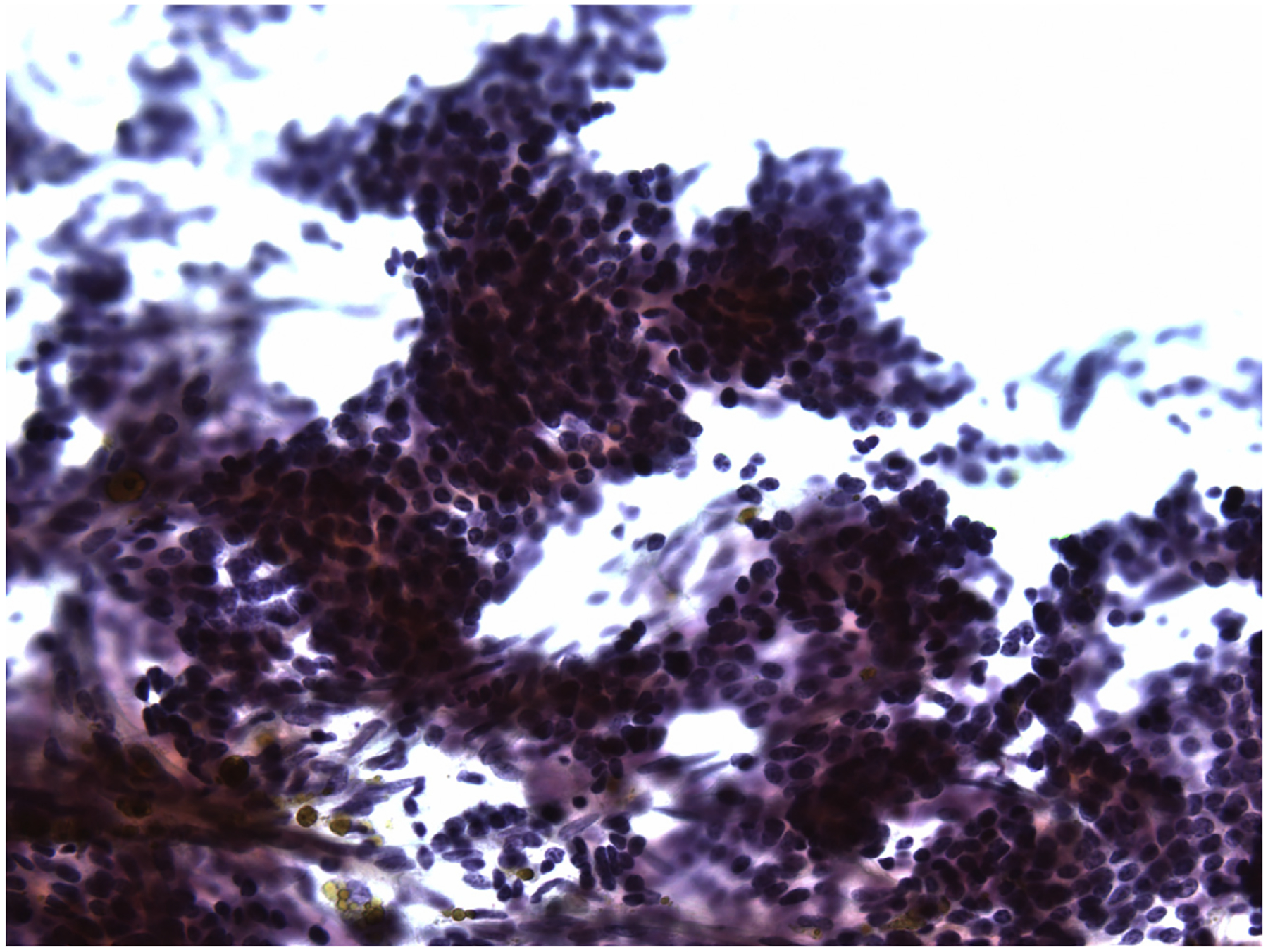
FNA of parathyroid adenoma. Cells are cohesive and have small amounts of eosinophilic cytoplasm and uniform round dark nuclei. The microscopic features overlap with those of a follicular neoplasm (Papanicolaou stain, original magnification ×400).
ONCOCYTIC/HÜRTHLE CELL NEOPLASM
Since the introduction of the term Hürthle cells to define thyroid follicular cells with abundant granular mitochondria-rich cytoplasm, their presence has been noticed in several conditions (Fig. 5),78–83 including adenomatous/hyperplastic nodules, chronic lymphocytic (Hashimoto) thyroiditis, multinodular goiter, but also Hürthle cell adenoma and carcinoma.80–82 A common diagnostic pitfall is misinterpreting the presence of a population of Hürthle cells in sheets admixed with background colloid with or without sheets of nononcocytic follicular cells78–84 as Hurthle cell neoplasm (HCN). As a general guideline, the presence of a mixture of Hürthle and non-Hürthle cells especially is more consistent with a benign nodule.
Fig. 5.
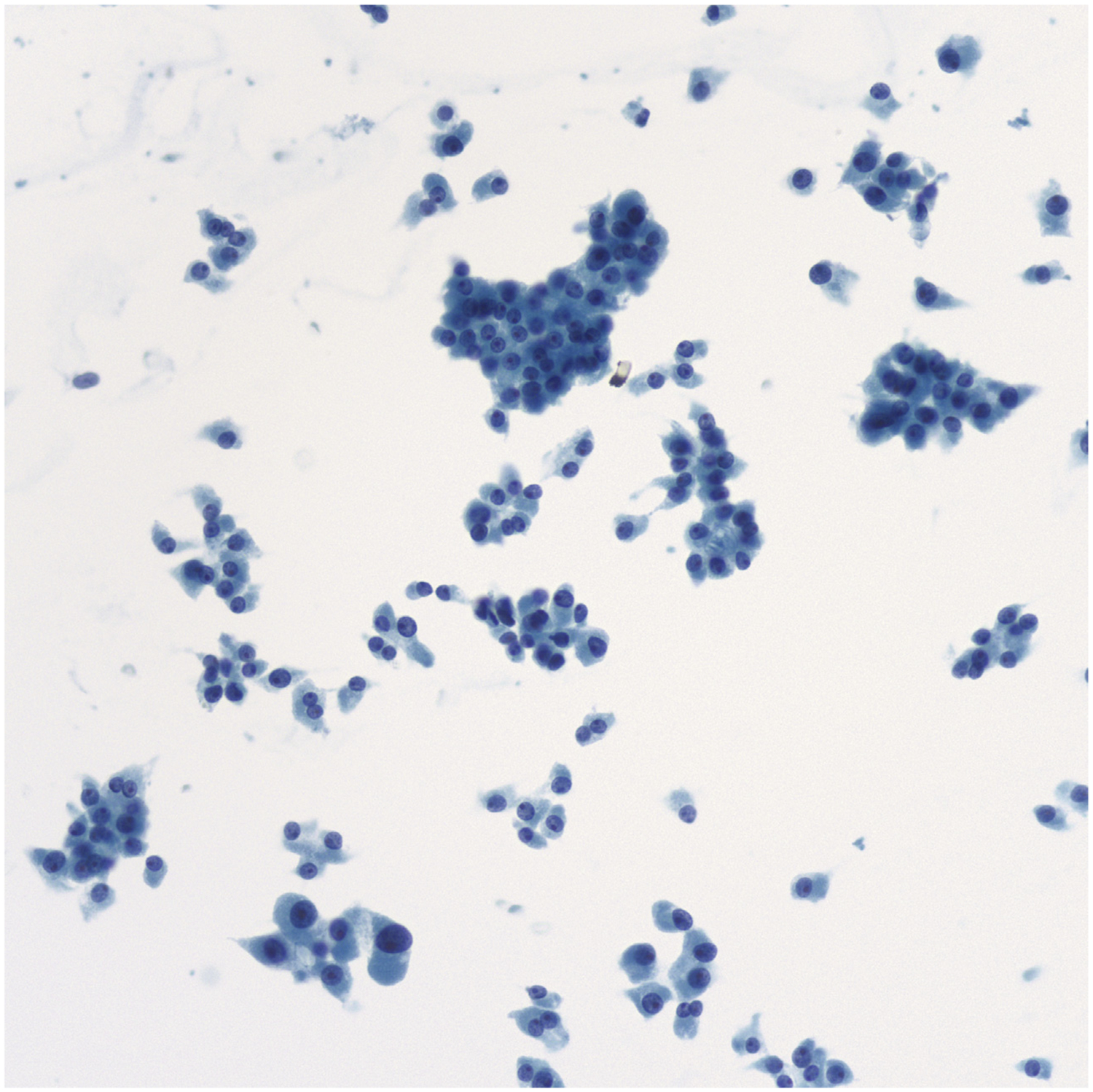
FNA of Hürthle cell neoplasm. Hürthle cells in sheets, small clusters, and single cells. The dispersed cells often have eccentrically placed nuclei with prominent nucleoli, but the nuclei lack the salt- and-pepper chromatin of MTC (Papanicolaou stain, original magnification ×400).
Most chronic LT cases can be recognized because of the predominance of lymphocytes compared with Hürthle cells. In difficult and controversial cases, mostly when the lymphocytic component is scant, a clue to the correct interpretation is based on finding oncocytic cells organized in small clusters of 3 to 10 cells with large nuclei, with or without glassy chromatin and with nuclear features that may raise concern for PTC. A high threshold should be maintained when background lymphocytes are present.84 However, in some cases the differential diagnosis between follicular neoplasm, Hürthle cell type (FNHCT) and the oncocytic variant of PTC is especially challenging and it may not be possible to distinguish between them. In some cases, the morphologic features of FNHCT resemble those of MTC. MTC are frequently composed of dispersed cells with eccentric nuclei and abundant dense granular cytoplasm.79–87 A subtle clue is that MTC nuclei do not show the widespread presence of prominent nucleoli characteristic of Hürthle cells. The application of an immunohistochemistry (IHC) panel, including thyroglobulin, calcitonin, carcinoembryonic antigen (CEA), and chromogranin can be useful. In addition, serum calcitonin levels are normal in cases of FNHCT.
The possibility of an intrathyroidal oncocytic parathyroid nodule (including both adenomas and carcinomas) represents another important cytologic pitfall.69,88 However, in contrast with FNHCT, oncocytic parathyroid samples are characterized by a monomorphic population of cells, with small round nuclei, and a more condensed chromatin pattern than in medullary carcinoma. A specific immunoprofile showing positivity for PTH, while being negative for thyroglobulin, calcitonin, and TTF-1, supports the diagnosis of a parathyroid neoplasm.
PAPILLARY THYROID CARCINOMA
PTC is the most common malignant tumor of the thyroid gland, with most having an indolent clinical course.89–94 When the classic nuclear features are present, they are specific for a diagnosis of PTC; however, when the cytologic features are limited, they can lead to diagnostic pitfalls, including both FP and FN results. Intranuclear cytoplasmic pseudoinclusions (INCIs) are seen in 50% to 100% of aspirates of PTC, depending on the specific PTC variant; however, INCIs are also found in several other benign and malignant entities, including MTC, poorly differentiated thyroid carcinoma (PDTC), ATC, hyalinizing trabecular tumor (HTT), noninvasive follicular thyroid neoplasm with papillarylike nuclear features (NIFTPs), and rarely benign nodules. For a definitive diagnosis of PTC, INCIs should always be interpreted in the context of other architectural and cellular features.1
Another characteristic morphologic feature of PTC is the longitudinal nuclear groove that is often seen in other entities such as oncocytic neoplasms, NIFTP, and some follicular adenomas. Even though most PTCs show scant colloid, occasional cases can have abundant colloid that can be misinterpreted as a benign thyroid nodule. Once again, careful evaluation of the combination of cellular and nuclear features is essential for making the correct interpretation.
One of the most challenging and common causes of an FP diagnosis of PTC is HTT.95,96 This rare tumor of follicular cell origin is characterized by trabecular growth, marked stromal hyalinization, and nuclear changes of PTC. Most HTTs are cytologically misinterpreted as PTC or suspicious for malignancy. However, the absence of papillary architecture, and elongated epithelial cells together with acellular stromal hyaline material are subtle microscopic clues of HTT. Ancillary studies that support the diagnosis of HTT include cytoplasmic positivity for MIB-1 and the lack of BRAFV600E mutation.
FOLLICULAR VARIANT OF PAPILLARY THYROID CARCINOMA AND NONINVASIVE FOLLICULAR THYROID NEOPLASM WITH PAPILLARYLIKE NUCLEAR FEATURES
The follicular variant of PTC (FVPTC) is completely or almost completely composed of a follicular architecture with atypical nuclear features of PTC.89–93 FVPTC is the most common variant of PTC, accounting for 15% to 30% of them and including 3 different subtypes: (1) infiltrative FVPTC (I-FVPTC); (2) encapsulated FVPTC (E-FVPTC), and (3) noninvasive encapsulated FVPTC (NI-EFVPTC).57–65 Although I-FVPTC frequently metastasizes to cervical lymph nodes similar to classic PTC, E-FVPTC behaves in indolent fashion, especially when there is no capsular or vascular invasion (NI-EFVPTC). The Endocrine Pathology Society working group (ESP-WG) reviewed a large series (n = 268) of NI-EFVPTCs and concluded that the absence of invasion was associated with an indolent biological behavior, similar to follicular adenomas, even when patients were treated conservatively with thyroid lobectomy without radioactive iodine therapy.60 Thus, the ESP-WG introduced the new diagnostic term for NI-FVPTC:NIFTP.60
Distinguishing between these 3 follicular-patterned subtypes is not possible by FNA.57–65 For this reason, a distinction between NIFTP and the other follicular-patterned neoplasms is problematic. Since the introduction of NIFTP, several studies have provided insight to the impact of this new terminology on the interpretation of thyroid lesions.57–65 If NIFTP was classified as a nonmalignant lesion, the ROM in each diagnostic category of TBSRTC would be reduced, particularly for nodules classified as indeterminate.57–65 Most NIFTP cases are classified in TBSRTC categories III, IV, and V (Fig. 6). Most importantly, to avoid the diagnostic pitfall of interpreting NIFTP as PTC by FNA, cases that are follicular patterned but lack papillary structures should not be diagnosed as malignant.
Fig. 6.
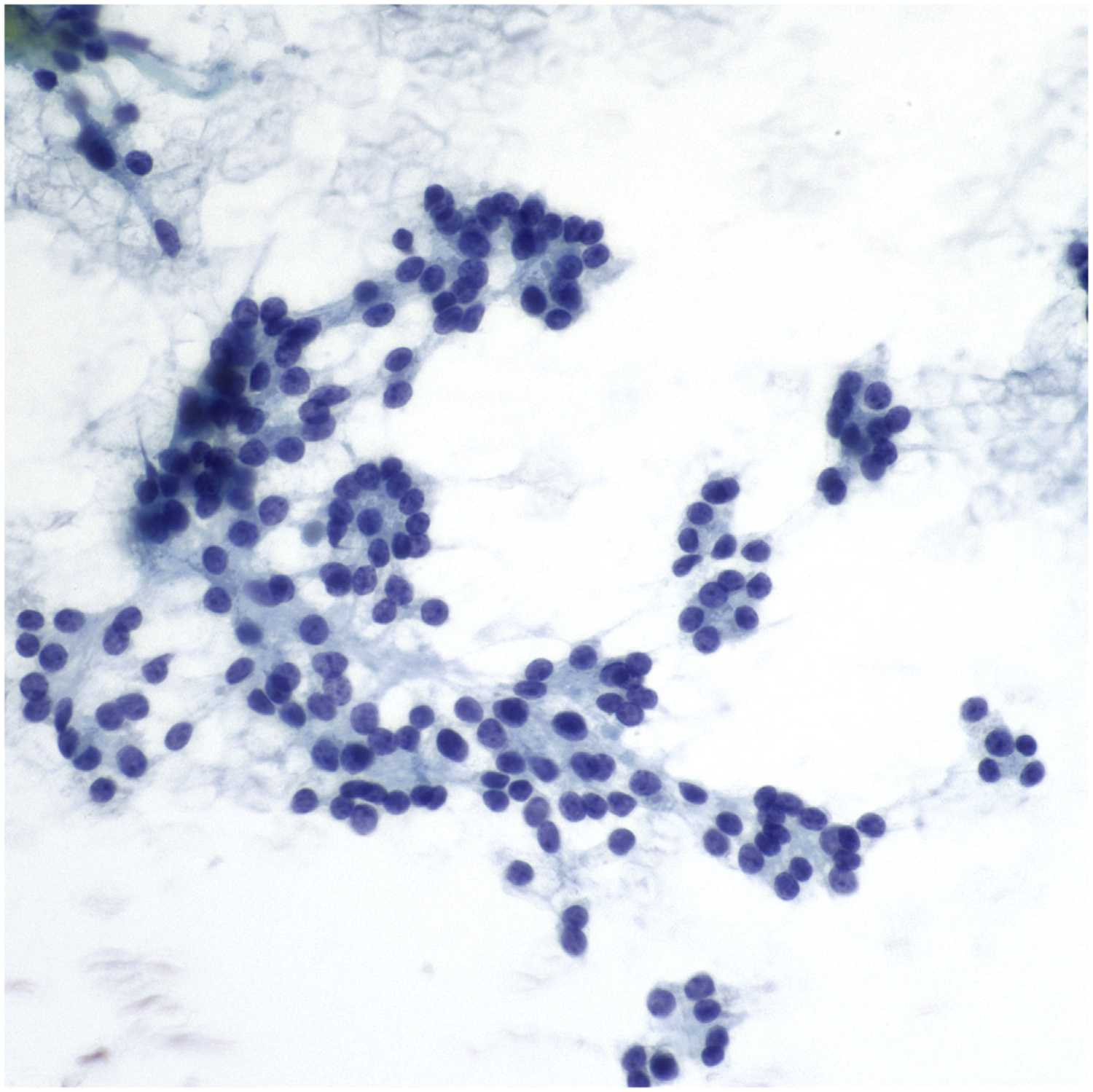
FNA of NIFTP. Epithelial cells show a follicular architectural arrangement with rare grooves and elongation. The diagnosis on the original cytology was follicular neoplasm (Bethesda category IV) (Papanicolaou stain, original magnification ×600).
MEDULLARY THYROID CARCINOMA
MTC accounts for 1% to 2% of thyroid cancers in the United States,97–101 and the FNA diagnosis of MTC can be challenging because of its many different cytomorphologic appearances. A recent meta-analysis from Trimboli and colleagues1,98,102 highlights the low sensitivity (56%) of FNA for diagnosing MTC. In some instances, the features of MTC, including loose clusters of cells showing round, polygonal, spindled, and plasmacytoid features, could be misdiagnosed as pattern of follicular neoplasm (Fig. 7). However, one of the most common pitfalls is mistaking MTC for an oncocytic (Hürthle cell) neoplasm. Although both entities can show cells with abundant granular oncocytic cytoplasm, MTC tends to lack the prominent nucleolus in most cells and has a more delicate salt-and-pepper chromatin than cells of oncocytic follicular neoplasm. As mentioned previously, intranuclear pseudoinclusions occur occasionally in MTC and can raise a differential diagnosis of PTC. However, careful attention to the nuclear qualities such as extensive nuclear grooves, oval shape, and powdery chromatin is needed, because they support a diagnosis of PTC. Another pitfall is that amyloid, identified in one-third of MTCs, can be indistinguishable from colloid, and it is also present in amyloid goiter. For FNA cases in which MTC is part of the differential diagnosis, application of an IHC panel, showing positive staining for synaptophysin, CEA, and calcitonin, or demonstration of an increased serum calcitonin level, can lead to the correct diagnosis.97–101
Fig. 7.
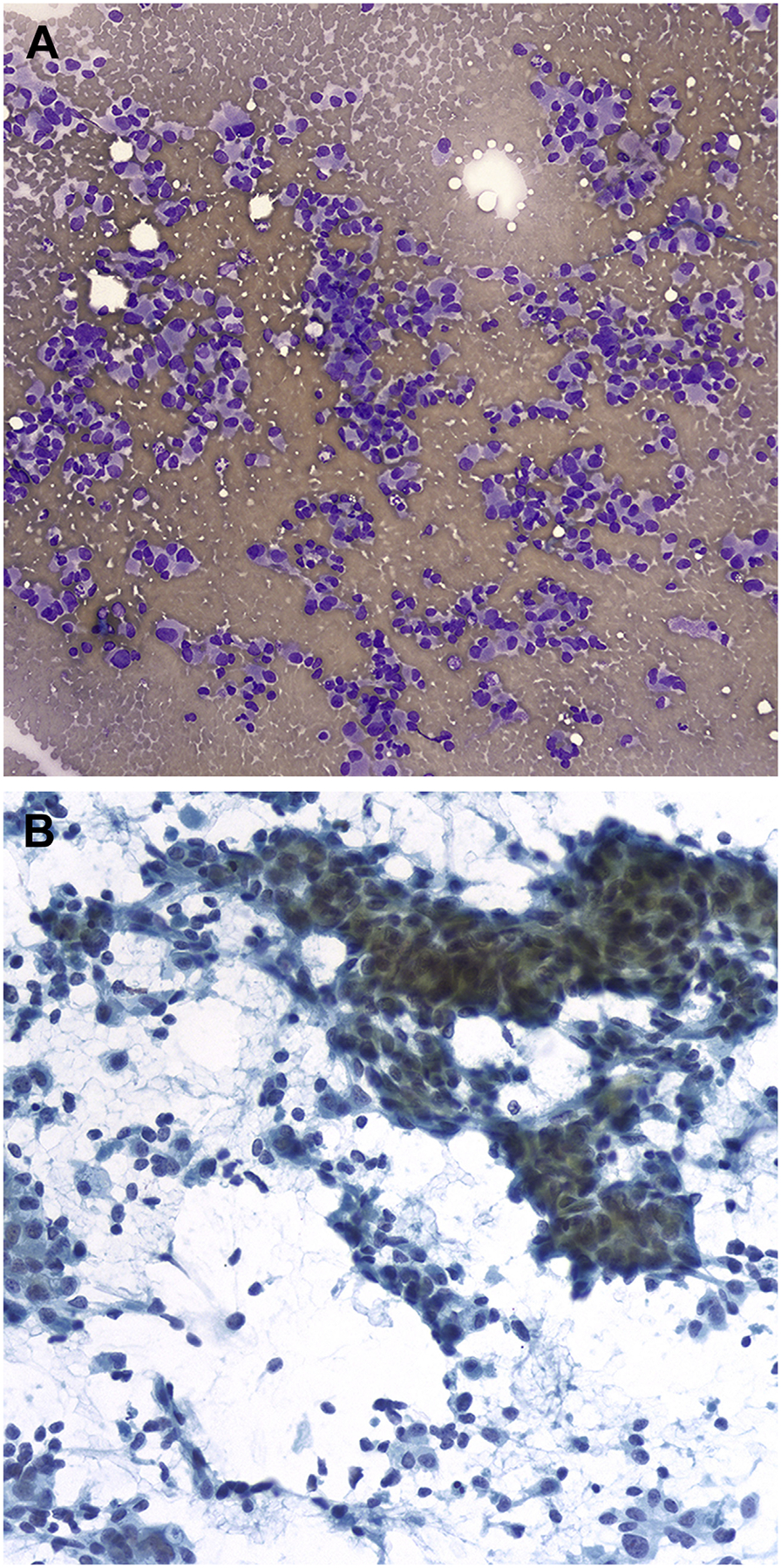
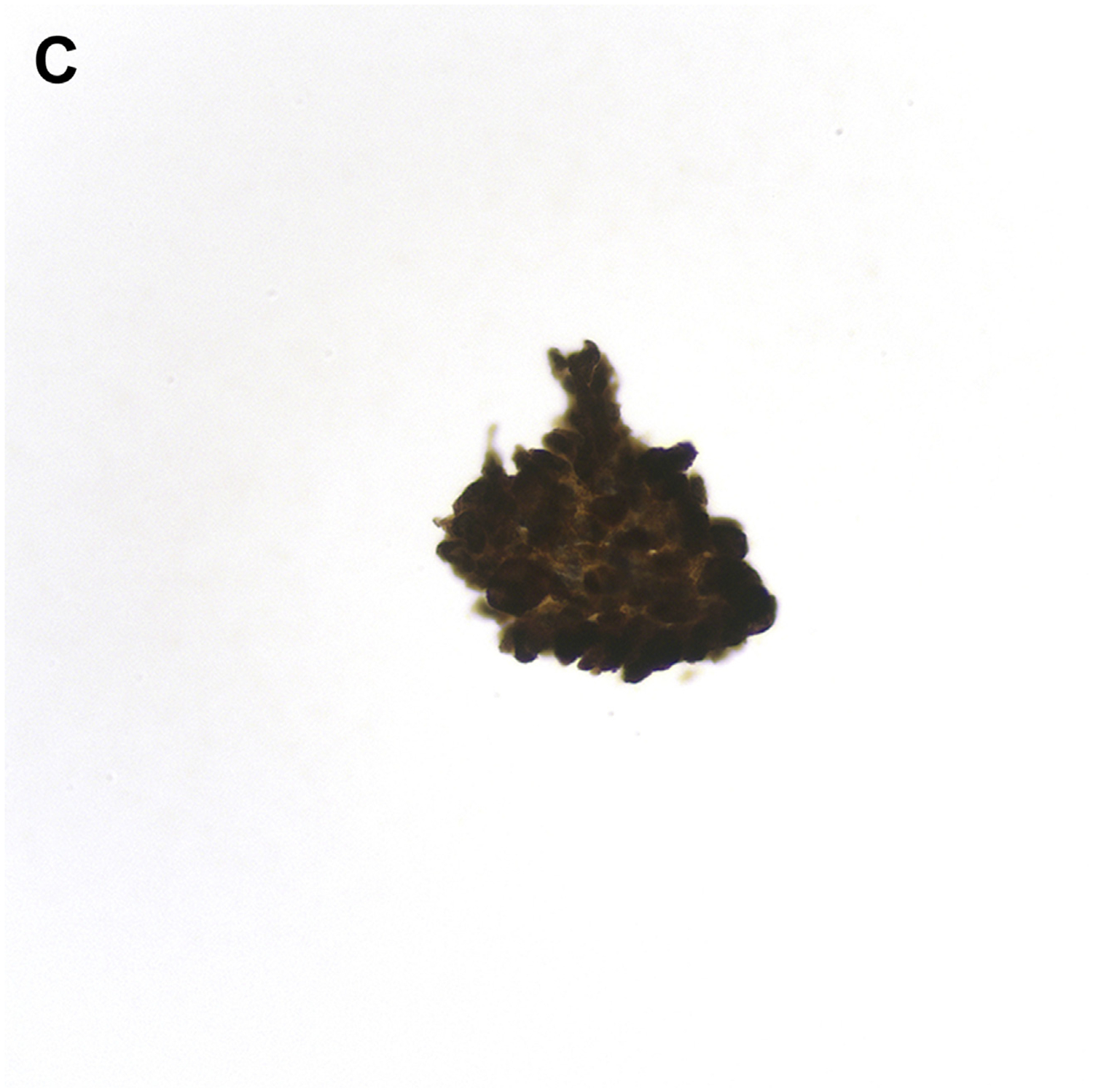
FNA of MTC. (A) Loose clusters and single cells with eccentrically placed nuclei (Diff-Quik stain, original magnification ×200). (B) The tumor shows spindling with a pseudopapillary appearance, nuclear elongation, and rare grooves. Single cells are also present in the background (Papanicolaou stain, original magnification ×400). (C) Tumor cells are immunochemically positive for calcitonin. (Avidin-Biotin complex method, original magnification ×400).
POORLY DIFFERENTIATED THYROID CARCINOMA
Poorly differentiated carcinoma (PDC) is a thyroid carcinoma composed of follicular cells organized in an insular, solid, or trabecular growth pattern.103–111 Cytologically, PDCs are difficult to recognize prospectively unless the case is associated with apoptosis, mitotic activity, and necrosis. The morphologic features of PDC overlap with those of follicular neoplasms; therefore, most of these cases are classified by FNA as SFN/FN. Nonetheless, insular and/or trabecular clusters of monomorphic atypical follicular cells with increased nuclear/cytoplasm ratio in a necrotic background might suggest a diagnosis of suspicious for malignancy not otherwise specified. Possible pitfalls include MTC, possible metastatic tumors, and lymphoproliferative disorders. The diagnosis of lymphoproliferative disorders is an important pitfall for those cases with predominance of isolated cells.105–111
ANAPLASTIC THYROID CARCINOMA AND METASTATIC TUMORS
ATC is an aggressive thyroid carcinoma, easily recognized in cytologic samples as a high-grade malignancy. Tumor cells are pleomorphic and can include both epithelioid and spindle features (Fig. 8).112–118 The cytomorphologic findings combined with characteristic clinical features, including the presence of a rapidly growing mass in a hard nodular thyroid gland, with infiltration into surrounding extrathyroid soft tissue, lead to the correct diagnosis. Given its high-grade undifferentiated appearance, the differential diagnosis includes metastatic malignant tumors, sarcoma, PDC, MTC, and lymphoma. The most common diagnostic pitfall is metastatic tumors, such as melanoma, sarcomatoid renal carcinoma, squamous cell carcinoma, and poorly differentiated adenocarcinoma of the lung.119–123 IHC studies, including PAX-8 and keratin, are useful when combined with clinical and radiological evidence of a tumor centered in the thyroid gland. Most anaplastic carcinoma are negative for TTF-1 and thyroglobulin, and a subset are negative for keratins. The possibility of a metastatic thyroid tumor should be considered, especially for those patients with a previous history of malignant extrathyroidal neoplasm (Fig. 9).
Fig. 8.
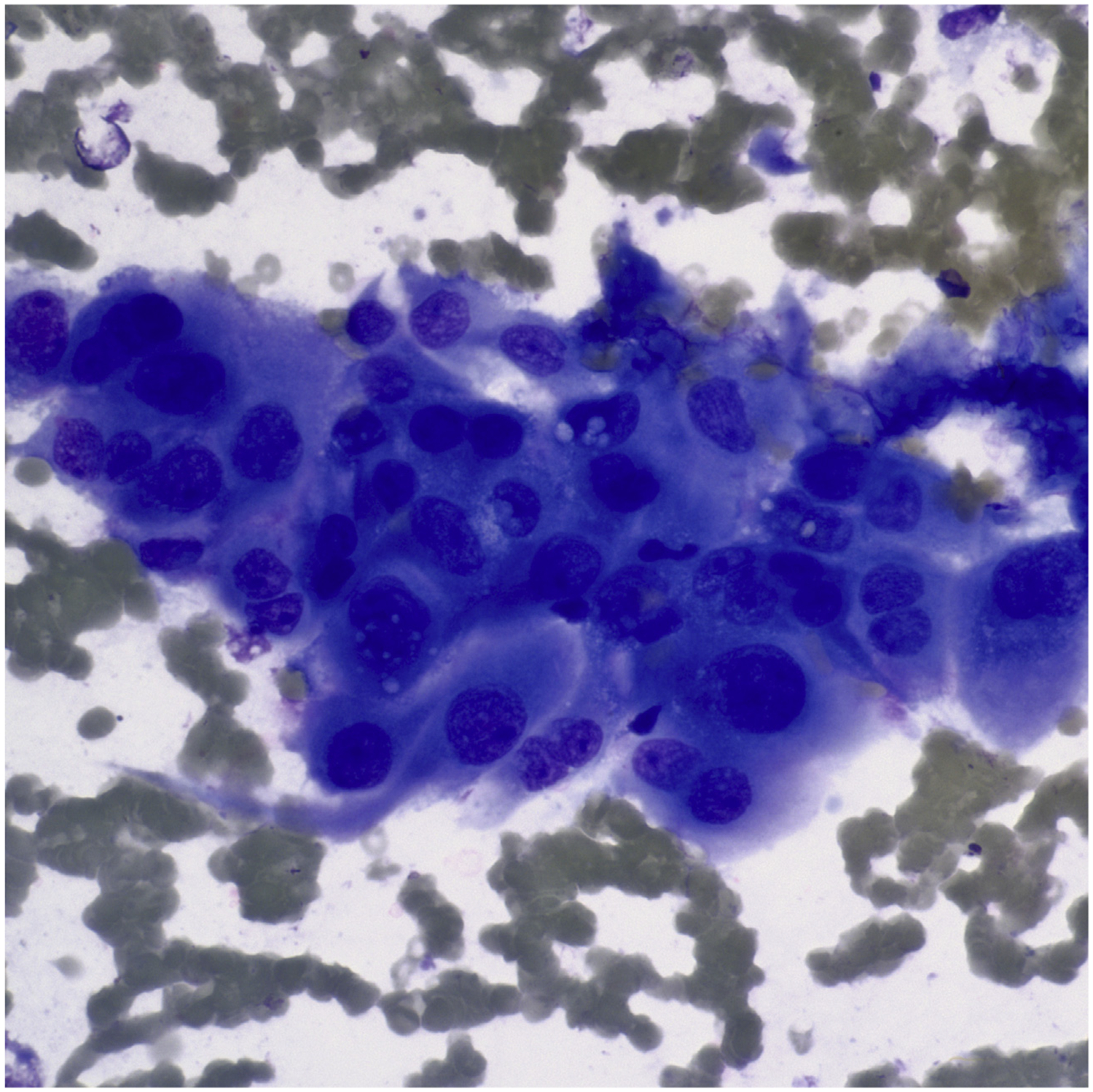
FNA of ATC showing a dispersed population of markedly atypical cells (Diff-Quik stain, original magnification ×600).
Fig. 9.
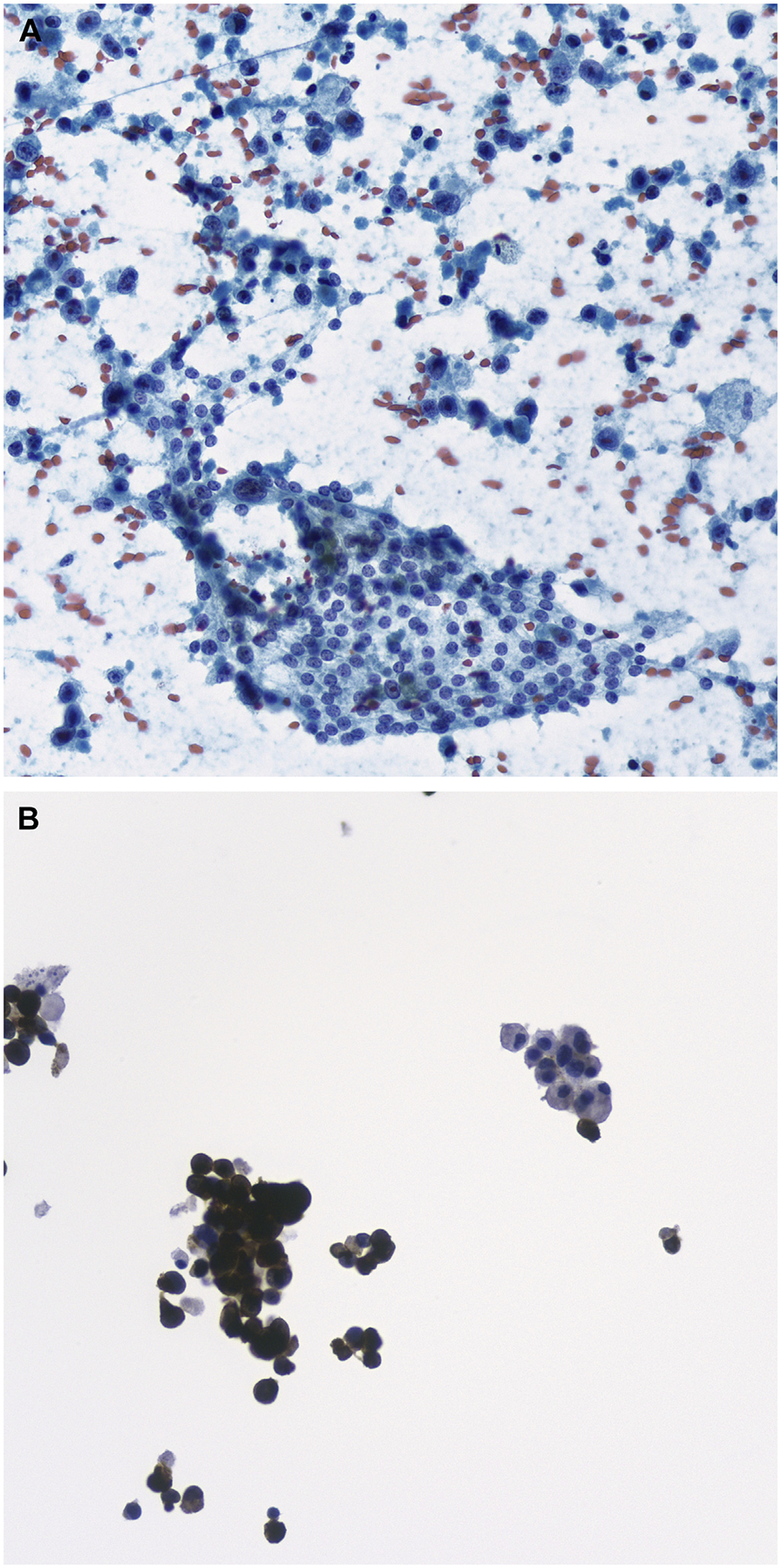
FNA of metastatic melanoma to the thyroid. (A) Infiltrating single cells of melanoma are seen in the background of nonneoplastic follicular cells (Papanicolaou stain, original magnification ×400). (B) Melan A immunochemical stain highlighting the tumor cells; the background normal follicular cells are negative. (Original magnification ×400).
SUMMARY
The main goal of thyroid FNA is to triage patients with nodules having a high ROM for surgery while avoiding unnecessary surgical procedures for others. The evaluation of patients with thyroid nodules should be based on a combination of the clinical, radiological, and cytomorphologic findings. Nonetheless, thyroid FNA is challenging and there are many potential diagnostic pitfalls, several of which are discussed in this article. An awareness of these potential diagnostic pitfalls combined with careful attention to cytologic and clinical features, along with judicious use of ancillary studies, can help to reduce errors and lead to more accurate FNA interpretations and improved patient care.
Footnotes
Conflicts of interest and funding: The authors have no conflicts of interest. This work did not receive any specific grant funding.
REFERENCES
- 1.Ali S, Cibas ES. The Bethesda system for reporting thyroid cytopathology Definitions, criteria and explanatory notes. 2nd edition Cham (Switzerland): Springer; 2018. [Google Scholar]
- 2.Tee YY, Lowe AJ, Brand CA, et al. Fine-needle aspiration may miss a third of all malignancy in palpable thyroid nodules: A comprehensive literature review. Ann Surg 2007;246(5):714–20. [DOI] [PubMed] [Google Scholar]
- 3.Cramer H Fine-needle aspiration cytology of the thyroid: an appraisal. Cancer 2000;90:325–9. [PubMed] [Google Scholar]
- 4.Gharib H, Papini E, Paschke R. Thyroid nodules: a review of current guidelines, practices and prospects. Eur J Endocrinol 2008;159:493–505. [DOI] [PubMed] [Google Scholar]
- 5.Ravetto C, Colombo L, Dottorini ME. Usefulness of fine-needle aspiration in the diagnosis of thyroid carcinomas. A retrospective study in 37,895 patients. Cancer Cytopathol 2000;90: 357–63. [PubMed] [Google Scholar]
- 6.Poller DN, Ibrahim AK, Cummings MH, et al. Fine-needle aspiration of the thyroid. Importance of an indeterminate diagnostic category. Cancer Cytopathol 2000;90:239–44. [DOI] [PubMed] [Google Scholar]
- 7.Rossi ED, Morassi F, Santeusanio G, et al. Thyroid fine-needle aspiration cytology processed by Thin Prep: An additional slide decreased the number of inadequate results. Cytopathology 2010;21:97–102. [DOI] [PubMed] [Google Scholar]
- 8.Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Fine-needle aspiration state-of-science Conference. Diagn Cytopathol 2008;36:425–37. [DOI] [PubMed] [Google Scholar]
- 9.Cross P, Chandra A, Giles T, et al. Guidance on the reporting of thyroid cytology specimens. London: The royal college of Pathologists; 2016. [Google Scholar]
- 10.Yang J, Schnadig V, Logrono R, et al. Fine needle aspiration of thyroid nodules: A study of 4703 patients with histologic and clinical correlations. Cancer 2007;111:306–15. [DOI] [PubMed] [Google Scholar]
- 11.Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med 2012;367:705–15. [DOI] [PubMed] [Google Scholar]
- 12.Tan YY, Kebebee E, Reiff E, et al. Does routine consultation of thyroid Fine needle aspiration cytology change surgical management? J Am Coll Surg 2007;205(1):8–12. [DOI] [PubMed] [Google Scholar]
- 13.Wong L, Baloch ZW. Analysis of the Bethesda system for reporting thyroid cytopathology and similar precursor thyroid cytopathology reporting schemes. Adv Anat Pathol 2012;19(5):313–9. [DOI] [PubMed] [Google Scholar]
- 14.Lew JI, Snyder RA, Sanchez YM, et al. Fine needle aspiration of the thyroid: correlation with final histopathology in a surgical series of 797 patients. J Am Coll Surg 2011;213:188–94. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014;159:676–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikiforova MN, Nikiforov Y. Molecular diagnostics and predictors in thyroid cancer. Thyroid 2009;19: 1351–61. [DOI] [PubMed] [Google Scholar]
- 17.Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab 2009;94:2092–8. [DOI] [PubMed] [Google Scholar]
- 18.Cheung CC, Carydis B, Ezzat S, et al. Analysis of RET/PTC gene rearrangements refines the fine needle aspiration diagnosis of thyroid cancer. J Clin Endocrinol Metab 2001;86:2187–90. [DOI] [PubMed] [Google Scholar]
- 19.Moses W, Weng J, Sansano I, et al. Molecular testing for somatic mutations improves the accuracy of thyroid fine needle aspiration biopsy. World J Surg 2010;34:2589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musholt TJ, Fottner C, Weber M, et al. Detection of papillary carcinoma by analysis of BRAF and RET/PTC1 mutations in fine needle aspiration biopsies of thyroid nodules. World J Surg 2010;34: 2595–603. [DOI] [PubMed] [Google Scholar]
- 21.Xing M BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 2007;28:742–62. [DOI] [PubMed] [Google Scholar]
- 22.Rorive S, D’Haene N, Fossion C, et al. Ultrasound-guided fine needle aspiration of thyroid nodules: stratification of malignancy risk using follicular proliferation grading, clinical and ultrasonographic features. Eur J Endocrinol 2001;162:1107–15. [DOI] [PubMed] [Google Scholar]
- 23.Bohacek L, Milas M, Mitchell J, et al. Diagnostic accuracy of surgeon-performed ultrasound guided fine needle aspiration of thyroid nodules. Ann Surg Oncol 2012;19:45–51. [DOI] [PubMed] [Google Scholar]
- 24.Piana S, Frasoldati A, Ferrari M, et al. Is a five-category reporting scheme for thyroid fine needle aspiration cytology accurate? Experience of over 18,000 FNAs reported at the same institution during 1998–2007. Cytopathology 2011;22:164–73. [DOI] [PubMed] [Google Scholar]
- 25.Nayar R, Ivanovic M. The indeterminate thyroid fine needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National cancer institute thyroid fine needle aspiration state of the science conference. Cancer 2009;117:195–2002. [DOI] [PubMed] [Google Scholar]
- 26.Carr R, Ustun B, Chhieng D, et al. Radiologic and clinical predictors of malignancy in the follicular lesion of undetermined significance of the thyroid. Endocr Pathol 2013;24:62–8. [DOI] [PubMed] [Google Scholar]
- 27.Jaragh M, Carydis VB, MacMillan C, et al. Predictors of malignancy in thyroid fine-needle aspirates “cyst fluid only” cases. Can potential clues of malignancy be identified? Cancer Cytopathol 2009; 120:305–10. [DOI] [PubMed] [Google Scholar]
- 28.Gage H, Hubbard E, Nodit L. Multiple squamous cells in thyroid fine needle aspiration: friends or foes? Diagn Cytopathol 2016;44(8):676–81. [DOI] [PubMed] [Google Scholar]
- 29.Faquin WC, Cibas E, Renshaw AA. Atypical cells in needle aspiration biopsy specimens of benign thyroid cysts. Cancer 2005;105:71–9. [DOI] [PubMed] [Google Scholar]
- 30.Jaffar R, Mohanty SK, Khan A, et al. Hemosiderin laden macrophages and hemosiderin within follicular cells distinguish benign follicular lesions from follicular neoplasms. Cytojournal 2009;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen AL, Renshaw AA, Faquin WC, et al. Thyroid FNA biopsies comprised of abundant, mature squamous cells can be reported as benign: A cytologic study of 18 patients with clinical correlation. Cancer Cytopathol 2018;126:336–41. [DOI] [PubMed] [Google Scholar]
- 32.Ryska A, Ludvíková M, Rydlová M, et al. Massive squamous metaplasia of the thyroid gland- report of three cases. Pathol Res Pract 2006;202: 99–106. [DOI] [PubMed] [Google Scholar]
- 33.Richmond BK, Judhan R, Chong B, et al. False-negative results with the Bethesda system of reporting thyroid cytopathology: Predictors of malignancy in thyroid nodules classified as benign by cytopathologic evaluation. Am Surg 2014;80(8): 811–6. [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson SR, Mandel S, LiVolsi VA, et al. Can cytomorphology differentiate between benign nodules and tumors arising in Graves’ disease? Diagn Cytopathol 2004;30(1):64–7. [DOI] [PubMed] [Google Scholar]
- 35.Haugen BR, Alexander E, Bible KC, et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belfiore A, Russo D, Vigneri R, et al. Graves’ disease, thyroid nodules and carcinoma. Clin Endocrinol 2001;55:711–8. [DOI] [PubMed] [Google Scholar]
- 37.Dobyns BM, Sheline GE, Workman JB, et al. Malignant and benign neoplasm of the thyroid in patients treated for hyperthyroidism: A report of the cooperative thyro-toxicosis therapy follow-up study. J Clin Endocrinol Metab 1974;38:976–98. [DOI] [PubMed] [Google Scholar]
- 38.Anila KR, Nayak Nn, Jayasree K. Cytomorphologic spectrum of lymphocytic thyroiditis and correlation between cytological grading and biochemical parameters. J Cytol 2016;33(3):145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatia A, Rajwanshi A, Dash RJ, et al. Lymphocytic thyroiditis-Is cytological grading significant? A correlation of grades with clinical biochemical, ultrasonographic and radionuclide parameters. Cytojournal 2007;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi KI, Ahn S, Park DY, et al. False-positive cytopathology results for papillary thyroid carcinoma: a trap for thyroid surgeons. Clin Otolaryngol 2017;42:1153–60. [DOI] [PubMed] [Google Scholar]
- 41.Boi F, Pani F, Mariotti S. Thyroid autoimmunity and thyroid cancer: review focused on cytological studies. Eur Thyroid J 2017;6(4):178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solano JG, Bascunana AG, Perez JS, et al. Fine needle aspiration of subacute granulomatous thyroiditis (De quervain’s thyroiditis): a clinic-cytologic review of 36 cases. Diagn Cytopathol 1997;16(3):214–20. [DOI] [PubMed] [Google Scholar]
- 43.Rosa M Cytologic features of subacute granulomatous thyroiditis can mimic malignancy in liquid-based preparations. Diagn Cytopathol 2016;44(8): 682–4. [DOI] [PubMed] [Google Scholar]
- 44.Anderson CE, Duvall E, Wallace WA. A single Thin-Prep slide may not be representative in all head and neck fine needle aspirate specimens. Cytopathology 2009;20:87–90. [DOI] [PubMed] [Google Scholar]
- 45.Vural C, Paksoy N, Gok ND, et al. Subacute granulomatous (De Quervain) thyroiditis: Fine needle aspiration cytology and ultrasonographic characteristics of 21 cases. Cytojournal 2015;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olah R, Hajos O, Soos Z, et al. De Quervain thyroiditis. Corner point of the diagnosis. Orv Hetil 2014; 155:676–80. [DOI] [PubMed] [Google Scholar]
- 47.Singer PA. Thyroiditis. Acute, subacute and chronic. Med Clin North Am 1991;75:61–77. [DOI] [PubMed] [Google Scholar]
- 48.Baloch ZW, LiVolsi VA. Follicular-patterned afflictions of the thyroid gland: Reappraisal of the most discussed entity in endocrine pathology. Endocr Pathol 2014;25:12–20. [DOI] [PubMed] [Google Scholar]
- 49.Renshaw A, Wang E, Wilbur D, et al. Interob-server agreement on microfollicles in thyroid fine needle aspirates. Arch Path Lab Med 2006;130: 148–52. [DOI] [PubMed] [Google Scholar]
- 50.Broome JT, Solorzano CC. The impact of atypia/follicular lesion of undetermined significance on the rate of malignancy in thyroid fine-needle aspiration: Evaluation of the Bethesda System for Reporting Thyroid Cytopathology. Surgery 2011;150: 1234–9. [DOI] [PubMed] [Google Scholar]
- 51.Shi Y, Ding X, Klein M, et al. Thyroid fine-needle aspiration with atypia of undetermined significance: a necessary or optional category? Cancer 2009;117:298–304. [DOI] [PubMed] [Google Scholar]
- 52.Damiani D, Suciu V, Vielh P. Cytopathology of follicular cell nodules. Endocr Pathol 2015;26:286–91. [DOI] [PubMed] [Google Scholar]
- 53.Nagarkatti SS, Faquin WC, Lubitz CC, et al. Management of thyroid nodules with atypical cytology on fine-needle aspiration biopsy. Ann Surg Oncol 2013;20:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olson MT, Clark DP, Erozan YS, et al. Spectrum of Risk of Malignancy in Subcategories of ‘Atypia of Undetermined Significance’. Acta Cytol 2011;55: 518–25. [DOI] [PubMed] [Google Scholar]
- 55.Horne MJ, Chhieng DC, Theoharis C, et al. Thyroid follicular lesion of undetermined significance: Evaluation of the risk of malignancy using the two-tier sub-classification. Diagn Cytopathol 2012;40: 410–5. [DOI] [PubMed] [Google Scholar]
- 56.Rossi ED, Martini M, Capodimonti S, et al. Morphology combined with ancillary techniques: An algorithm approach for thyroid nodules. Cytopathology 2018;29(5):418–27. [DOI] [PubMed] [Google Scholar]
- 57.Rossi ED, Bizzarro T, Martini M, et al. Cytopathology of Follicular Cell Nodules. Adv Anat Pathol 2017;24(1):45–55. [DOI] [PubMed] [Google Scholar]
- 58.Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2009;9: 1159–65. [DOI] [PubMed] [Google Scholar]
- 59.Baloch Z, LiVolsi VA, Henricks WH, et al. Encapsulated follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 2002;118(4):603–5. [PubMed] [Google Scholar]
- 60.Howitt BE, Chang S, Eslinger M, et al. Fine-needle aspiration diagnoses of noninvasive follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 2015;144(6):850–7. [DOI] [PubMed] [Google Scholar]
- 61.Ganly I, Wang L, Tuttle MR, et al. Invasion rather than nuclear features correlates with outcome in encapsulated follicular tumors: further evidence for the reclassification of the encapsulated papillary thyroid carcinoma follicular variant. Hum Pathol 2015;46(5):657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2016;2(8):1023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson LD. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: a name change to noninvasive follicular thyroid neoplasm with papillary like nuclear features would help to prevent overtreatment. Mod Pathol 2016; 29(7):698–707. [DOI] [PubMed] [Google Scholar]
- 64.Bizzarro T, Martini M, Capodimonti S, et al. The morphologic analysis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) on liquid based cytology: Some insights of their identification in our institutional experience. Cancer Cytopathol 2016;124(10):699–710. [DOI] [PubMed] [Google Scholar]
- 65.Faquin W, Wong L, Afrogheh A, et al. Impact of reclassifying non invasive FVPC on the risk of malignancy in the Bethesda system for reporting thyroid Cytopathology. Cancer Cytopathol 2016; 124(3):181–7. [DOI] [PubMed] [Google Scholar]
- 66.Nikiforov Y, Baloch ZW, Hodak SP, et al. Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillary like nuclear features. JAMA Oncol 2018. 10.1001/jamaoncol.2018.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohori NP, Wolfe J, Carty S, et al. The influence of the noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) resection diagnosis on the false-positive thyroid cytology rate relates to quality assurance thresholds and the application of NIFTP criteria. Cancer Cytopathol 2017;125:692–700. [DOI] [PubMed] [Google Scholar]
- 68.Agarwal AM, Bentz JS, Hungerford R, et al. Parathyroid fine needle aspiration cytology in the evaluation of parathyroid adenoma: cytologic findings from 53 patients. Diagn Cytopathol 2009;37: 407–10. [DOI] [PubMed] [Google Scholar]
- 69.Rossi ED, Mulè A, Zannoni GF, et al. Asymptomatic intrathyroidal parathyroid adenoma. Report of a case with a cytologic differential diagnosis including thyroid neoplasms. Acta Cytol 2004; 48(3):437–40. [DOI] [PubMed] [Google Scholar]
- 70.Cho M, Oweity T, Brandler TC, et al. Distinguishing parathyroid and thyroid lesions on ultrasoundguide fine needle aspiration: A correlation of clinical data, ancillary studies and molecular analysis. Cancer Cytopathol 2017;125(9):674–82. [DOI] [PubMed] [Google Scholar]
- 71.Vanderlaan PA, Marqusee E, Krane JF. Usefulness of diagnostic qualifiers for thyroid fine-needle aspirations with atypia of undetermined significance. Am J Clin Pathol 2011;136(4):572–7. [DOI] [PubMed] [Google Scholar]
- 72.Krane JF, Vanderlaan PA, Faquin WC, et al. The atypia of undetermined significance/follicular lesion of undetermined significance: malignant ratio: a proposed performance measure for reporting in The Bethesda System for thyroid cytopathology. Cancer Cytopathol 2012;120(2):111–6. [DOI] [PubMed] [Google Scholar]
- 73.Ohori NP, Nikiforova MN, Schoedel KE, et al. Contribution of molecular testing to thyroid fine needle aspiration cytology of “Follicular lesion of undetermined significance/Atypia of undetermined significance”. Cancer Cytopathol 2010;118:17–23. [DOI] [PubMed] [Google Scholar]
- 74.Colanta A, Lin O, Tafe L, et al. BRAF mutation analysis of fine-needle aspiration biopsies of papillary thyroid carcinoma: impact on diagnosis and prognosis. Acta Cytol 2011;55:563–9. [DOI] [PubMed] [Google Scholar]
- 75.Nikiforov YE, Ohori P, Hodack SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metabol 2011;96: 3390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Radkay L, Chiosea SI, Seethala RR, et al. Thyroid nodules with KRAS mutations are different from nodules with NRAS and HRAS mutations with regard to cytopathologic and histopathologic outcome characteristics. Cancer Cytopathol 2014; 122:873–82. [DOI] [PubMed] [Google Scholar]
- 77.Rossi ED, Martini M, Capodimonti S, et al. BRAF (V600E) mutation analysis on LBC-processed aspiration biopsies predicts bilaterality and nodal involvement in papillary thyroid microcarcinoma. Cancer Cytopathol 2013;121:291–7. [DOI] [PubMed] [Google Scholar]
- 78.Giorgadze T, Rossi ED, Fadda G, et al. Does the fine-needle aspiration diagnosis of “Hürthle-cell neoplasm/follicular neoplasm with oncocytic features” denote increased risk of malignancy? Diagn Cytopathol 2004;31(5):307–12. [DOI] [PubMed] [Google Scholar]
- 79.Straccia P, Rossi ED, Bizzarro T, et al. A meta-analytic review of the Bethesda System for Reporting Thyroid Cytopathology: Has the rate of malignancy in indeterminate lesions been underestimated? Cancer Cytopathol 2015;123(12):713–22. [DOI] [PubMed] [Google Scholar]
- 80.Rossi ED, Martini M, Straccia P, et al. The cytologic category of oncocytic (Hurthle) cell neoplasm mostly includes low-risk lesions at histology: an institutional experience. Eur J Endocrinol 2013; 169(5):649–55. [DOI] [PubMed] [Google Scholar]
- 81.Montone KT, Baloch ZW, LiVolsi VA. The thyroid Hurthle (oncocytic) cell and its associated pathologic conditions: a surgical pathology and cytopathology review. Arch Pathol Lab Med 2008;132: 1241–50. [DOI] [PubMed] [Google Scholar]
- 82.Kasper KA, Stewart J, Das K. Fine-needle aspiration cytology of thyroid nodules with hurthle cells: cytomorphologic predictors for neoplasms, improving diagnostic accuracy and overcoming pitfalls. Acta Cytol 2014;58:145–52. [DOI] [PubMed] [Google Scholar]
- 83.Díaz Del Arco C, Fernández Aceñero MJ. Preoperative Diagnosis of Neoplastic or Malignant Hürthle Cell Lesions: A Chimera? Acta Cytol 2018;62(3): 193–203. [DOI] [PubMed] [Google Scholar]
- 84.Moreira AL, Waisman J, Cangiarella JF. Aspiration cytology of the oncocytic variant of papillary adenocarcinoma of the thyroid gland. Acta Cytol 2004;48(2):137–41. [DOI] [PubMed] [Google Scholar]
- 85.Maximo V, Rios E, Sobrinho-Simoes M. Oncocytic lesions of the thyroid, kidney, salivary glands, adrenal cortex and parathyroid glands. Int J Surg Pathol 2014;22:33–6. [DOI] [PubMed] [Google Scholar]
- 86.Bai S, Baloch ZW, Samulski TD, et al. Poorly differentiated oncocytic (hürthle cell) follicular carcinoma: an institutional experience. Endocr Pathol 2015;26(2):164–9. [DOI] [PubMed] [Google Scholar]
- 87.Rossi ED, Mule ‘ A, Miraglia A, et al. Granular cell tumour on conventional cytology and thin-layer smears. Cytopathology 2005;16(5):259–61. [DOI] [PubMed] [Google Scholar]
- 88.Wong YP, Sharifah NA, Tan GC, et al. Intrathyroidal oxyphilic parathyroid carcinoma: A potential diagnostic caveat in cytology? Diagn Cytopathol 2016; 44(8):688–92. [DOI] [PubMed] [Google Scholar]
- 89.Khanafshar E, Lloyd RV. The spectrum of papillary thyroid carcinoma variants. Adv Anat Pathol 2011; 18:90–7. [DOI] [PubMed] [Google Scholar]
- 90.Albores-Saavedra J, Wu J. The many faces and mimics of papillary thyroid carcinoma. Endocr Pathol 2006;17:1–18. [DOI] [PubMed] [Google Scholar]
- 91.Baloch Z, LiVolsi VA, Tondon R. Aggressive variants of follicular cell derived thyroid carcinoma; the so called “Real Thyroid Carcinomas”. J Clin Pathol 2013;66:733–43. [DOI] [PubMed] [Google Scholar]
- 92.Rosai J, Carcangiu ML, DeLellis RA. Tumors of the thyroid gland Vol 3rd series, fascicle 5. Washington, DC: Armed forces institute of pathology. [Google Scholar]
- 93.Llyod RV, Osamura RY, Kloppel G, et al. Tumors of the thyroid gland In: WHO Classifications of tumors of the endocrine organs. 4th edition; 2017. [Google Scholar]
- 94.Hawn WA. The many appearances of papillary carcinoma of the thyroid. Cleve Clin Q 1976;43: 207–16. [DOI] [PubMed] [Google Scholar]
- 95.Saglietti C, Piana S, La Rosa S, et al. Hyalinizing trabecular tumor of the thyroid: Fine needle aspiration cytological diagnosis and correlation with histology. J Clin Pathol 2017;70:641–7. [DOI] [PubMed] [Google Scholar]
- 96.Choi WJ, Baek JH, Ha EJ, et al. The ultrasonography features of hyalinizing trabecular tumor of the thyroid gland and the role of fine needle aspiration cytology and core needle biopsy in its diagnosis. Acta Radiol 2015;56(9):1113–8. [DOI] [PubMed] [Google Scholar]
- 97.Wells SA, Asa S, Dralle H, et al. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. The American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Thyroid 2015; 25:567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trimboli P, Treglia G, Guidobaldi L, et al. Detection rate of FNA cytology in medullary thyroid carcinoma: A meta-analysis. Clin Endocrinol 2015;82: 280–5. [DOI] [PubMed] [Google Scholar]
- 99.Papaparaskeva K, Nagel H, Droese M. Cytologic diagnosis of medullary carcinoma of the thyroid gland. Diagn Cytopathol 2000;22:351–8. [DOI] [PubMed] [Google Scholar]
- 100.Pusztaszeri M, Bongiovanni M, Faquin WC. Update on the cytological and molecular features of medullary thyroid carcinoma. Adv Anat Pathol 2014;21: 26–35. [DOI] [PubMed] [Google Scholar]
- 101.Rossi ED, Raffaelli M, Mulè A, et al. Relevance of immunocytochemistry on thin-layer cytology in thyroid lesions suspicious for medullary carcinoma: a case-control study. Appl Immunohistochem Mol Morphol 2008;16(6):548–53. [DOI] [PubMed] [Google Scholar]
- 102.Baloch ZW, LiVolsi VA. Special types of thyroid carcinoma. Histopathology 2018;72(1):40–52. [DOI] [PubMed] [Google Scholar]
- 103.Carcangiu ML, Zampi G, Rosai J. Poorly differentiated (insular) thryoid carcinoma. A reinterpretation of Langahns “ wuchernde struma”. Am J Surg Pathol 1984;8:655–68. [DOI] [PubMed] [Google Scholar]
- 104.Volante M, Landolfi S, Chiusa L, et al. Poorly differentiated carcinomas of the thyroid with trabecular insular, and solid pattern: A clinicopathological study of 183 patients. Cancer 2004;100:950–7. [DOI] [PubMed] [Google Scholar]
- 105.Bongiovanni M, Bloom l, Krane JF, et al. Cytomorphologic features of poorly differentiated thyroid carcinoma: a multi-institutional analysis of 40 cases. Cancer 2009;117:185–94. [DOI] [PubMed] [Google Scholar]
- 106.Kane SV, Sharma TP. Cytologic diagnostic approach to poorly differentiated thyroid carcinoma: A single-institution study. Cancer Cytopathol 2015;123:82–91. [DOI] [PubMed] [Google Scholar]
- 107.Barwad A, Dey P, Nahar Saikia UN, et al. Fine needle aspiration cytology of insular carcinoma of thyroid. Diagn Cytopathol 2012;40:E40–7. [DOI] [PubMed] [Google Scholar]
- 108.Khetrapal S, Rana S, Jetley S, et al. Poorly differentiated carcinoma of thyroid: Case report of an uncommon entity. J Cancer Res Ther 2018;14(5): 1142–4. [DOI] [PubMed] [Google Scholar]
- 109.Purkait S, Agarwal S, Mathur SR, et al. Fine needle aspiration cytology features of poorly differentiated thyroid carcinoma. Cytopathology 2016;27(3): 176–84. [DOI] [PubMed] [Google Scholar]
- 110.Sironi M, Collini P, Cantaboni A. Fine needle aspiration cytology of insular thyroid carcinoma. A report of four cases. Acta Cytol 1992;36(3):435–9. [PubMed] [Google Scholar]
- 111.Laforga JB, Cortés VA. Oncocytic poorly differentiated (insular) thyroid carcinoma mimicking metastatic adenocarcinoma. A case report and review of the literature. Diagn Cytopathol 2019. 10.1002/dc.24147. [DOI] [PubMed] [Google Scholar]
- 112.Bauman ME, Tao LC. Cytopathology of papillary carcinoma of the thyroid with anaplastic transformation. A case report. Acta Cytol 1995;39(3): 525–9. [PubMed] [Google Scholar]
- 113.Oktay MH, Smolkin MB, Williams M, et al. Metastatic anaplastic carcinoma of the thyroid mimicking squamous cell carcinoma: report of a case of a challenging cytologic diagnosis. Acta Cytol 2006; 50(2):201–4. [DOI] [PubMed] [Google Scholar]
- 114.Maatouk J, Barklow TA, Zakaria W, et al. Anaplastic thyroid carcinoma arising in long-standing multinodular goiter following radioactive iodine therapy: report of a case diagnosed by fine needle aspiration. Acta Cytol 2009;53(5):581–3. [DOI] [PubMed] [Google Scholar]
- 115.Feng G, Laskin WB, Chou PM, et al. Anaplastic thyroid carcinoma with rhabdoid features. Diagn Cytopathol 2015;43(5):416–20. [DOI] [PubMed] [Google Scholar]
- 116.Suh HJ, Moon HJ, Kwak JY, et al. Anaplastic thyroid cancer: ultrasonographic findings and the role of ultrasonography-guided fine needle aspiration biopsy. Yonsei Med J 2013;54(6):1400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guarda LA, Peterson CE, Hall W, et al. Anaplastic thyroid carcinoma: cytomorphology and clinical implications of fine-needle aspiration. Diagn Cytopathol 1991;7(1):63–7. [DOI] [PubMed] [Google Scholar]
- 118.Mehdi G, Ansari HA, Siddiqui SA. Cytology of anaplastic giant cell carcinoma of the thyroid with osteoclast-like giant cells-a case report. Diagn Cytopathol 2007;35(2):111–2. [DOI] [PubMed] [Google Scholar]
- 119.Straccia P, Mosseri C, Brunelli C, et al. Diagnosis and Treatment of Metastases to the Thyroid Gland: a Meta-Analysis. Endocr Pathol 2017;28(2): 112–20. [DOI] [PubMed] [Google Scholar]
- 120.Papi G, Fadda G, Corsello SM, et al. Metastases to the thyroid gland: prevalence, clinicopathological aspects and prognosis: a 10-year experience. Clin Endocrinol (Oxf) 2007;66(4):565–71. [DOI] [PubMed] [Google Scholar]
- 121.Rossi ED, Martini M, Straccia P, et al. Is thyroid gland only a “land” for primary malignancies? role of morphology and immunocytochemistry. Diagn Cytopathol 2015;43(5):374–80. [DOI] [PubMed] [Google Scholar]
- 122.Ciobanu D, Vulpoi C, Găluşcă B, et al. The value of the immunohistochemical exam in the diagnosis of the secondary malignant tumors to the thyroid gland. Rom J Morphol Embryol 2007;48(2):113–9. [PubMed] [Google Scholar]
- 123.Shah SS, Faquin WC, Izquierdo R, et al. FNA of misclassified primary malignant neoplasms of the thyroid: Impact on clinical management. Cytojournal 2009;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]


