Abstract
Background
COVID‐19 has been spreading worldwide with a significant death toll. Solid‐organ transplantation (SOT) recipients are at higher risk due to their suppressed immune system. In this study, we aimed to conduct a systematic review on COVID‐19 clinical manifestations and treatment strategies in SOT recipients.
Methods
We searched three databases for relevant terms related to COVID‐19 and transplantation. 50 studies, including 337 patients, were reviewed.
Results
Two hundred thirty six patients were male, with a mean age of 49.9 years. The most prevalent group was the kidney 57.0%, followed by 17.2% heart and 13.6% liver. Fever and cough were the most reported clinical presentations. Infiltration (55.4%) in chest x‐ray and ground‐glass opacity (67.1%) in CT scans were the most radiological findings. It was found that 96.8% and 72.4% of patients present with CRP level and lymphocytopenia, respectively, and 70.6% of kidney recipients patients presented with high creatinine levels. The most common baseline immunosuppressants were calcineurin inhibitors (88.9%) and antimetabolites (73.2%). Antimetabolites (84.3%) and calcineurin inhibitors (54.3%) were discontinued/decreased 84.3% whereas glucocorticoids dosage almost has no change (77.9%) or even increased. 18.4% of cases had died, and 65.9% were discharged.
Conclusions
Patients' demographics, signs, symptoms, and radiographic findings in SOT recipients are almost similar to the general population. However, gastrointestinal symptoms appear to be more common. There are different treatment strategies, but in most of them, antimetabolite and calcineurin inhibitors were decreased or discontinued, while corticosteroids were increased. Finally, COVID‐19 seems to be more severe and has higher mortality in SOT recipients compared to the general population.
Keywords: clinical manifestation, COVID‐19, radiography, systematic review, transplantation, treatment
1. INTRODUCTION
Coronavirus disease (COVID‐19) is caused by a single‐stranded RNA virus called severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The first human case was reported in Wuhan, Hubei province of China in December 2019, and by a few weeks, the COVID‐19 infection has the characteristics of a “pandemic”. 1
According to the World Health Organization (WHO), fever, fatigue, and dry cough are the most common symptoms of COVID‐19. Patients may also experience shortness of breath, myalgia, sore throat, and gastrointestinal (GI) symptoms. Real‐time polymerase chain reaction (RT‐PCR) is the most accurate detection method by now; however, some centers have chosen chest x‐ray (CXR) or computerized tomography (CT) investigations as first‐line triage tools due to long RT‐PCR turnaround times. 2 , 3
There are several threats for transplant recipients, but one particularly significant threat is emerging of infectious diseases. There have been several new viral diseases since 1980, including HIV, SARS‐CoV, West Nile Virus, Influenza A/H1N1, Zika, Ebola, and now COVID‐19. 4 , 5
Since the outbreak, there is increasing evidence that those with existing comorbidities, older age, or a compromised immune system are at higher risk of developing severe and even fatal respiratory diseases. Solid‐organ transplant (SOT) recipients are also considered to be in this risk group, especially as they get treated with immune‐suppressive drugs. 3 , 6 It is also assumed that transplant recipients may have a more significant viral burden and shedding, resulting in higher infectivity and potential spread to other individuals, including healthcare professionals. Moreover, they have shown atypical clinical manifestations and worse prognosis in comparison to the general population; hence, different treatment approaches may be needed. 7 , 8 , 9 , 10
COVID‐19 has immediately and dramatically impacted the world. Considering the early nature of the pandemic, knowledge about COVID‐19 and its impact on SOT patients is limited to case reports and experts’ discussions. 11 In the present study, we aimed to perform a thorough systematic review aiming at collecting any clinical evidence accrued to date on the impact of COVID‐19 on SOT recipients.
2. METHODS
2.1. Study design and search strategies
The present study was conducted along with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement, and it has been listed in the International Prospective Register of Systematic Reviews (PROSPERO) database (ID CRD42020181465). A comprehensive literature review of PubMed, Embase, and Scopus entries between December 1, 2019 and May 22, 2020 was conducted for relevant terms related to COVID‐19 and SARS‐CoV2 in transplant patients and setting (Appendix 1).
2.2. Study selection
First, two reviewers (A.M, AH) independently screened the title and abstracts of all eligible studies. Inclusion criteria for studies were the description of SOT recipients who got infected with COVID‐19. Excluding criteria were pediatric transplantation, guidelines, unavailable full texts, and lack of sufficient data. Non‐English publications were translated into English, using Google's translation service. If eligibility was indeterminable, the full text was considered, and disagreements were resolved by consensus. Of all articles, 50 were regarded as relevant to this review. 3 , 7 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 PRISMA flow chart of study selection is shown in Figure 1.
FIGURE 1.
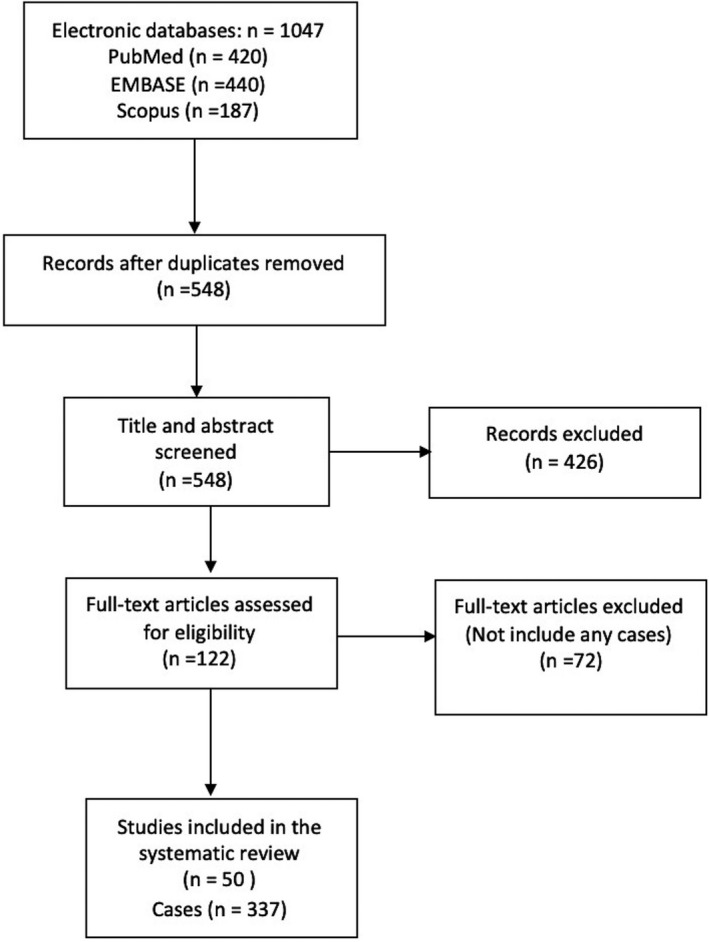
PRISMA flowchart of literature search
2.3. Data extraction
Three authors (A.M, S.M, AH) then separately extracted data from all relevant manuscripts using a pre‐defined data extraction sheet. Parameters such as patients' demographics including age, sex, country of residency, comorbidities, past medical history including the transplanted organ and post‐transplant time, baseline immunosuppressant therapy regimen, COVID‐19 clinical manifestations (Signs, Symptoms), intensive care unit (ICU) admission and intubation, abnormal laboratory data (WBC count, C‐reactive protein (CRP) serum level and serum Creatinine level (Cr), RT‐PCR tests, reported radiographic findings, and patients’ outcome were extracted.
2.4. Statistical analysis
The statistical analysis was performed using SPSS version 21.0 (SPSS Inc). Continuous variables were displayed as mean ± standard deviation, and categorical variables were reported as counts and percentages.
3. RESULTS
3.1. Description of included studies
We found 1047 references by applying the search strategy in the databases. We then discarded 499 duplicate citations. 426 articles were also excluded due to apparent irrelevancy of their topics in primary screening (Figure 1). In the secondary screening of 122 full texts, we excluded 72 papers. Finally, 50 studies, including 36 case reports, and 14 case series which overall have presented 337 patients were included in this systematic review. 3 , 7 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 The studies of Qin, L et al and Zhong, z et al reported a same case; to avoid duplication bias, we excluded (Qin, L et al) from our review. Table 1 has summarized the studies and patients' characteristics.
Table 1.
Summary of all included articles
| Author | Cases | country | Gender | Age | TX. Organ | Outcome |
|---|---|---|---|---|---|---|
| Abrishami, A. et al 12 | 12 | Iran | 9 males, 3 females | 47.6 (Mean) | Kidney | 8 expired, 4 recovered |
| Aigner, C. et al 13 | 1 | Germany | Female | 59 | Lung | Recovered |
| Alberici, F. et al 14 | 20 | Italy | 16 males, 4 females | 59 (Mean) | Kidney | 5 expired, 12 hospitalized, 3 discharged |
| Arpali, E et al 15 | 1 | Turkey | Female | 28 | Kidney | Recovered |
| Banerjee, D. et al 16 | 7 | England | 4 males, 3 females | 57.42 (mean) | Kidney | 1 expired, 2 recovered, 2 hospitalized, 2 intubated |
| Bartiromo, M et al 7 | 1 | Italy | Female | 36 | Kidney | Recovered |
| Billah, M. et al 17 | 1 | USA | Male | 44 | Kidney | Hospitalized |
| Bin, L et al 18 | 1 | China | Male | 50 | Liver | Recovered |
| Bussalino, E et al 19 | 1 | Italy | Male | 32 | Kidney | Recovered |
| Chen, S et al 20 | 1 | China | Male | 49 | Kidney | Recovered |
| Cheng, D. R. et al 78 | 2 | China | 1 male, 1 female | 56.5 (Mean) | Kidney | Recovered |
| Cozzi, E. et al 21 | 2 | Italy | 2 males | 58.5 (Mean) | Lung | 1 expired, 1 recovered |
| de Barros Machado, D. J. et al 22 | 1 | Brazil | Male | 69 | Liver‐kidney | Recovered |
| Donato, M. F. et al 23 | 8 | Italy | 6 males, 2 females | 63(Mean) | Liver | 6 recovered, 2 hospitalized |
| Farfour, E. et al 24 | 1 | France | Male | 35 | Lung | Not stated |
| Fernandez‐Ruiz, M et al 25 | 18 | Spain | 14 males, four females | 66.33 (Mean) | 8 kidneys, 6 livers, 4 hearts | 5 Expired, 4 hospitalized, 1 intubated, 8 recovered |
| Fontana, F. et al 26 | 1 | Italy | Male | 61 | Kidney | Recovered |
| Gandolfini, I et al 27 | 2 | Italy | 1 male, 1 female | 63.5 (Mean) | Kidney | 1 expired, 1 recovered |
| Gao, F. et al 28 | 3 | China | 3 males | 48.6 (Mean) | Liver | 1 expired, 2 recovered |
| Guillen, E et al 29 | 1 | Spain | Male | 50 | Kidney | Intubated |
| Hammami, M. B. et al 30 | 1 | USA | Male | 63 | Liver | Recovered |
| Holzhauser, L. et al 31 | 2 | USA | 1 male, 1 female | 67 (Mean) | Heart | 1 expired, 1 recovered |
| Hsu, J. et al 32 | 1 | USA | Male | 39 | Kidney‐Heart | Recovered |
| Huang J et al 33 | 1 | China | Male | 58 | Kidney | Expired |
| Johnson, K et al 34 | 1 | USA | Male | 57 | Kidney | Recovered |
| Kates, O et al 35 | 4 | USA | 3 males, 1 female | 62 (Mean) | 1 kidney, 1 liver, 1 heart, 1 lung | Recovered |
| Ketcham, S. et al 36 | 13 | USA | 13 males | 61 (Mean) | 10 hearts, 2 heart‐kidney, 1 heart‐lung | 2 expired, 2 hospitalized, 9 recovered |
| Kim, Y et al 37 | 2 | Korea | 2 males | 46 (Mean) | Kidney | Recovered |
| Kocak, B. et al 38 | 2 | Turkey | 2 females | 41.5 (Mean) | kidney | Recovered |
| Koczulla, R. A. et al 39 | 1 | Germany | Female | 64 | Lung | Recovered |
| Latif, F. et al 40 | 28 | USA | 22 males, 6 females | 64.0 (Median) | Heart | 7 expired, 4 hospitalized, 17 recovered |
| Li, F. et al 79 | 2 | China | 2 males | 47 (Mean) | Heart | Recovered |
| Marx, D et al 41 | 1 | France | Male | 58 | Kidney | Recovered |
| Mathies, D et al 42 | 1 | Germany | Male | 77 | Heart | Recovered |
| Meziyerh, S et al 43 | 1 | The Netherlands | Male | 35 | Kidney | Recovered |
| Montagud‐Marrahi, E et al 44 | 33 | Spain | 19 males, 14 females | 57.3 (Mean) | Kidney | 2 expired, 2 hospitalized, 28 recovered |
| Nair, V. et al 45 | 10 | USA | 6 males, 4 females | 56.3 (Mean) | Kidney | 3 expired, 7 recovered |
| Namazee, N. et al 46 | 1 | Iran | Female, | 63 | Kidney | Expired |
| Ning, L et al 80 | 1 | China | Male | 29 | Kidney | Recovered |
| Pereira, M et al 47 | 90 | USA | 53 male, 37 female | 57 (Median) | 46 kidneys, 17 lung, 13 livers, 9 heart, 3 heart‐kidney, 1 liver‐kidney,1 pancreas‐kidney | 16 expired, 15 hospitalized, 59 recovered |
| Seminari, E et al 48 | 1 | Italy | Male | 50 | Kidney | Recovered |
| Travi, G. et al 49 | 13 | Italy | 9 males, 4 females | 59 (Median) | 4 kidneys, 7 livers, 2 heart‐kidney | 3 expired, 10 not stated |
| Tschopp, J. et al 50 | 21 | Switzerland | 15 males, 6 females | 56 (Median) | 10 kidney, 5 liver, 2 kidney‐pancreas, 1 kidney‐lung, 1 pancreas, 1 lung, 1 heart | 2 expired, 3 hospitalized, 16 recovered |
| Wang, J et al 51 | 1 | China | Male | 49 | Kidney | Recovered |
| Xuan, T. M. et al 52 | 1 | China | Male | 70 | Lung | Recovered |
| Zhang, H et al 53 | 5 | China | 4 males, 1 female | 44.8 (Mean) | Kidney | 3 recovered, 2 hospitalized |
| Zhang, M, et al et al 54 | 1 | China | Male | 49 | Kidney | Recovered |
| Zhong, Z et al 55 | 2 | China | 2 Males | 42.5 (Mean) | Liver, Kidney | Recovered |
| Zhu, L.; Gong, N et al 56 | 10 | China | 8 males, 2 females | 45 (Mean) | Kidney | 1 expired, 8 recovered, 1 hospitalized |
| Zhu, L; Xu, X et al 57 | 1 | China | Male | 52 | Kidney | Recovered |
3.2. Demographic data and comorbidities
A total of 337 patients who met the criteria were included in the current systematic review. More than two‐thirds (236 patients) were male, and 101 were female. Regarding the available clear data, the mean ± SD age of patients was 49.9, with a range of 24 to 80 years in different studies. The most prevalent patient group was the kidney transplant recipients with a population of 192 (57.0%), followed by 58 (17.2%) with heart, 46(13.6%) with liver, 25 (7.4%) with lung, eight (2.4%) with heart‐kidney, three (0.9%) with pancreas‐kidney, one (0.3%) with heart‐kidney, and one (0.3%) with lung‐kidney, with an average of 10.6 (0.2‐31) years transplant history among the clearly reported data. Forty‐six cases were defined with their donor status; fourteen (30.4%) cases were mentioned that had a living donor, and 32 (69.6%) got their transplanted organ from deceased donors.
one hundred sixty four patients were mentioned to have hypertension, 114 diabetes mellitus, and 10 malignancies. The patients were under medication for these underlying diseases, such as antihypertensive or antihyperglycemic treatments. All patients were under immunosuppressive treatment due to history of transplantation; calcineurin inhibitors were used by 88.9% of patients; it was followed by glucocorticoids for 62.8% of patients. The baseline immunosuppressive treatment of patients is summarized in Table 2.
Table 2.
Summary of available immunosuppressant therapies
| Baseline treatment frequency (%) | Treatment changes after COVID‐19 infection | Newly prescribed (number of reported cases) | |||
|---|---|---|---|---|---|
| Total available Cases = 325 | Not Changed** | Decreased or discontinued** | Increased** | ||
| Glucocorticoids | 204 (62.8%) | 113/145 (77.9%) | 6/145 (4.1%) | 26/145 (17.9%) | 21 |
| Antimetabolites | |||||
| Mycophenolate mofetil | 227 (69.8%) | 33/210 (15.7%) | 177/210 (84.3%) | 0/210 (0%) | 0 |
| Azathioprine or Mizoribine | 11 (3.4%) | ||||
| Calcineurin inhibitors | 289 (88.9%) | 139/256 (54.3%) | 116/256 (45.3%) | 1/256 (0.4%) | 3 |
| Immunoglobulin‐based therapies | 7 (2.1%) | 2/2 (100%) | 0/2 (0%) | 0/2 (0%) | 41 |
| mTOR Inhibitor | 22 (6.8%) | 2/18 (11.1%) | 16/18 (88.9%) | 0/18 (0%) | 0 |
| Cept (TNF Inhibitor—Belatacept) | 6 (1.8%) | Data are only available about one discontinued case | |||
Number of cases with the change status/total case under the regimen as their baseline treatment (with pre‐ and post‐COVID‐19 infection available data).
3.3. Primary signs and symptoms, and diagnostic measures (RT‐PCR, radiographic findings, and laboratory data)
The most reported clinical presentation was fever (228 cases) followed by cough (181 cases). The frequency of reported sign/symptoms is shown in Figure 2.
FIGURE 2.
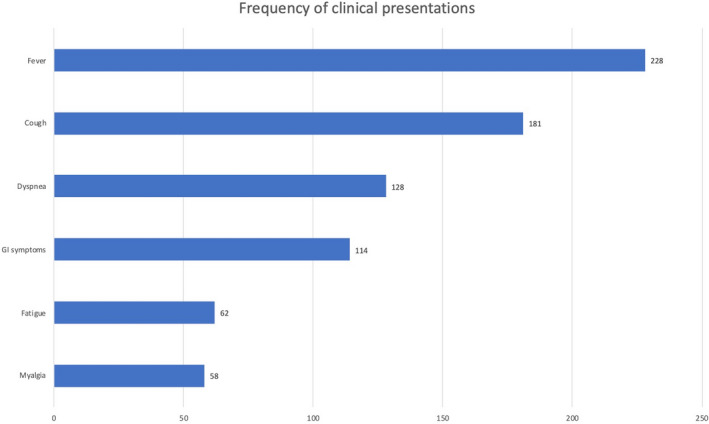
Most common signs and symptoms
It was stated that 209 RT‐PCR tests were done for the studied patients; all had a positive PCR test except one with a negative test who was diagnosed via CT scan. 189 cases were evaluated through CXR, and there were 21 cases of normal CXR, while 168 (88.9%) were not; among these abnormal cases, 74 cases were reported with details of findings that included 41 (55.4%) cases of infiltrations, 15 (20.3%) cases of consolidations, 11 (14.7%) cases of opacities, 2 (2.7%) cases of haziness, and 5 (6.8%) cases with other findings.
Chest CT scan is mentioned to be performed for 76 cases, and 68 were reported with details. Sixty‐seven (98.5%) patients were reported to have abnormal findings; 45 (67.1%) cases were reported with ground‐glass opacity (GGO), 8 (11.9%) with mixed patterns, 3 (4.5%) with viral infection presentation, 2 (3.0%) with patchy consolidation, and one (1.5%) with regular patterns. Eight cases (11.9%) were reported with other findings.
Among the studies that had reported the definite laboratory results of CRP or lymphocyte counts, 72.4% (71/98) of cases were reported with lymphocytopenia and 96.8% (92/95) with an increased CRP. Among the 68 cases of kidney transplant with available creatinine (Cr) level data, 48 (70.6%) had presented with high Cr level.
3.4. Treatment strategies
Seventy‐nine of 291 reported cases, (27.1%) patients were reported to have an ICU admission, and 85/239 (35.6%) cases were intubated. The most discontinued/decreased therapies were antimetabolites 177/210 (84.3%), while the highest number of newly prescribed treatments was immunoglobulin‐based therapies with 41 cases. In 4.1% of the patients who were using glucocorticoids, it was stopped using or the dosage was decreased, and in 77.9%, the dosage was not changed. The baseline and final immunosuppressant therapies with their changes for patients are summarized in Table 2.
Twenty cases (6.7%) were reported with IVIG prescription post‐COVID‐19 infection. Hydroxychloroquine was prescribed for 200/ 297 (67.3%) of patients, 137/ 221 (62.0%) consumed antibiotics, and 118/ 224 (52.7%) received other treatments, including 222 Lopinavir/Ritonavir (LPV/r) and two cases of unknown antiviral.
3.5. Outcomes
Sixty‐one (18.4%) cases were reported to be expired among all the studied patients with reported outcomes (333 cases). 218 (65.9%) patients were discharged, and 54 (16.3%) were reported by the Authors to be still hospitalized.
The available mean ± SD age of expired patients was 61.3 ± 11.7 years, and the available sex proportion was 17/7 for male to female. Excluding studies, in which their data about expired patients were not clear in details, the mortality rate was 18/79 (22.8%), 3/21 (14.3%), 2/10 (20.0%), and 1/6 (16.7%) among patients with kidney, liver, heart, and lung transplantations, respectively; among these 18 expired patients with kidney transplantation, high creatinine level was noted in 14 of 16 (85.7%) cases with available reports.
4. DISCUSSION
The infection caused by SARS‐CoV‐2 was declared as a global pandemic by WHO. Previously, other coronaviruses caused epidemic outbreaks such as SARS‐CoV and Middle East respiratory syndrome coronavirus (MERS‐CoV) were also seen in SOT recipients. 58 , 59 Considering the fact that they belong to high‐risk groups due to their immunosuppressive medications, it is vital to determine COVID‐19 manifestations in them.
In our study, nearly two‐thirds of the patients were male. Also, the mean age was around 50. A recent meta‐analysis of more than 2500 COVID‐19 patients showed a mean age of about 52 years and the male proportion of 56%. 60 This finding shows that there is no noticeable difference in the mean age of COVID‐19 patients between the general population and transplant patients. It is also reported that male patient proportion was greater than women in a case series study of 5700 COVID‐19 patients from New York City. 61 However, the higher male proportion of COVID‐19 in transplant recipients might be due to the higher male proportion in transplant patients based on the previous studies. 62
According to Global observatory on donation and transplantation, kidney, liver, heart, and lung are the most frequently transplanted organs in the world 63 ; however, the published cases from heart recipients seem to be more than liver recipients. Besides, 164 patients were reported to have hypertension, and 114 have diabetes mellitus. Comparing the data to the normal population as reported in Yang, J et al, the normal hosts of COVID‐19 represented hypertension, diabetes mellitus, cardiovascular, and respiratory system disease as the most prevalent comorbidities, which is consistent with our results. 64
The reported clinical manifestations in the reviewed studies are quite variable. Manuel, O et al 65 reported that transplant recipients often present with mild or atypical symptoms, and sometimes fever may be absent. However, our total result matches the same clinical profile of the general population with the most common clinical symptoms of fever, cough, and dyspnea. 66 Nevertheless, it should be noted that about one‐third of the studied patients presented with GI symptoms. A recently published, multicenter study of 204 patients with confirmed COVID‐19 suggests that approximately 18.5% of patients presented with GI symptoms, including diarrhea, vomiting, or abdominal pain. 67 Therefore, SOT patients may suffer from GI symptoms more often than the general population.
Since SOT patients are on medications that modulate the inflammatory response, symptoms associated with community‐acquired respiratory viruses are typically less severe and less frequent than in regular healthy hosts, especially in patients with severe lymphocytopenia. 68 We found that about 72% of the patients with available data show lymphocytopenia in their blood tests. Tan, Li et al 69 suggest that lymphocytopenia is an effective and reliable indicator of the severity and hospitalization in COVID‐19 patients. Furthermore, in our review, among the patients with reported CRP, almost 97% have shown high plasma concentration. Wang L. found that CRP levels were positively correlated with lung lesions and disease severity and, it can be a factor to consider for estimating the prognosis. 70
Among the 68 cases of kidney transplant with available Cr level data, nearly 70% had presented with high Cr level. This may be due to acute kidney injury (AKI) in COVID‐19 infection. It is reported that AKI in COVID‐19 can accompany by sepsis, multi‐organ failure, and shock, suggesting the cause of AKI can be due to acute tubular necrosis (ATN). 71 Furthermore, Cheng Y et al 72 found that AKI is a predictor of mortality in COVID‐19 infection, and previously, it was reported that kidney injury was associated with an increased chance of death in patients with H1N1 and SARS as well.
Our study shows that nearly 89% of the patients had shown abnormality in their CXR, and the most frequent findings were infiltrations, consolidations, opacities, and haziness. Wong, HYF et al 2 reported that 65% of the confirmed COVID‐19 cases had abnormal CXR findings, and consolidation was the most common one. This can indicate that SOT recipients have shown more abnormality in their CXR and this can be used for primary screening for the disease by seeing infiltrations or consolidations. Besides, our chest CT evaluations demonstrate abnormalities in about 98% of patients; GGO in 67% of cases, 12% mixed, 4.5% with viral infection presentation, and 3% with patchy consolidation which is consistent with a case series of 81 patients with COVID‐19 from the general population. 73 However, Ju, CR et al reported that the imaging demonstrations of COVID‐19 in SOT patients do not share standard features with the majority of the general population. 3
Calcineurin inhibitors, antimetabolites, and corticosteroids are most frequently used as baseline immunosuppressants; however, in COVID‐19‐confirmed cases, antimetabolites were mostly ceased whereas prescription of corticosteroids was kept in treatment or even increased in dosage. It was considered essential to use appropriate doses of corticosteroids throughout the process, as it could suppress inflammatory storms and promote the recovery from pneumonia without severe side effects as described in Zhong, Z et al 55 Furthermore, Fix, O.K et al suggest that the immune response may be the main reason for pulmonary injury due to COVID‐19, and immunosuppression may be protective. 74 However, the World Health Organization recommends avoiding corticosteroids for treatment of COVID‐19 unless for another therapeutic purpose. 75 Overall, use of steroids is still controversial and the efficacy has not been proven in clinical trials in the general population or in transplant recipients specifically. 20 In addition, it is a common strategy when treating pneumonia caused by opportunistic virus infections to reduce or even discontinue immunosuppressant drugs to allow recipients the opportunity to reacquire anti‐infection immunity within a short period in post‐transplant settings. 57 Besides, IVIG was administered to 20 patients. Jawhara, S 76 reported that immunotherapy with IgG combined with antiviral medications could provide an alternative treatment against COVID‐19 infection. These immune IgG antibodies collected from the healed patients will boost the immune response in newly infected patients.
This study shows that almost 27% of the patients were admitted to ICU, and around 36% were intubated. Besides, Richardson, S et al 61 study on patients with COVID‐19 admitted to different hospitals in New York City showed that 14.2% of the patients were hospitalized in ICU, 12.2% underwent invasive ventilation, and almost 21% died. However, Cascella, M et al 77 suggest that the death rate ranges from 1% to 2% depending on the study and country. Comparing ICU admission and intubation can indicate that the course of the disease is more severe in SOT patients than in the normal hospitalized population. In addition, our results explicate that the mortality is around 18% among all patients. It is important to note that many of these SOT patients who contracted COVID‐19 may still be in hospitals, some even in the ICU, and the outcome is still not predictable. These findings show that SOT patients are at a higher risk of mortality than the general population but lower in comparison with normal hospitalized patients as mentioned in Richardson, S et al. 61
4.1. Limitations and suggestions on future study
To best of our knowledge, this is the only systematic review on SOT recipients with COVID‐19 that has evaluated the different aspects of the disease from diagnosis, to treatment modifications. The emergence of the outbreak and lack of high‐level evidence in the reviewed literature makes this study much more critical, which may shed light on the better management of transplant patients, who should be considered as a vulnerable group during this pandemic.
There are several limitations to making comparisons, generalizations, or concluding the currently reported cases of SOT patients treated for COVID‐19. These recently published cases present a considerable variation in transplant and clinical characteristics as well as treatment strategies. Most of the currently published cases do not describe patients’ incubation periods, and days of hospital stay thoroughly to allow for comparison. Additionally, very limited data regarding medication interactions and reasons for changing some drugs were available. Besides, given the fact that a significant number of individuals may not present with symptoms until the disease progresses to advanced stages, the overall generalizability of results and conclusions may somewhat be hampered by publication bias. Another limitation was that performing pooled data analyses was impossible due to the lack of information from the studies retrieved and their design, which are mostly case study and series. At the time of writing this study, many cases were still at the hospital, some even intubated, and the reported mortality can be higher since the outcome of these patients is not completely reported yet. In addition, it is too soon for evaluation of the long‐term effects of immunosuppressant discontinuation or dose reduction in SOT patients on graft rejection, and there is an essential need for more comprehensive clinical studies including short‐ and long‐term follow‐up cohort assessments. Also, more concentration is needed on prolonged viral shedding since there is a potential of putting the lives of other patients and health workers at risk.
AUTHOR CONTRIBUTION
Seyed Ali Moosavi and Amirali Mashhadiagha designed the study, collected the data, and drafted the manuscript. Alireza Hashemazar contributed to design the study, collected the data, participated in data analyses, and participated in drafting the manuscript. Amir Human Hoveidaei analyzed the data and participated in drafting the manuscript. Nasrin Motazedian, and Davide Bolignano contributed to design the study, participated in drafting the manuscript, reviewed the manuscript, and edited the final version.
ACKNOWLEDGEMENTS
This work is supported by Shiraz University of Medical Sciences, Shiraz, Iran (Grant ID 99‐01‐15‐22506).
Appendix 1.
Search strategy
Embase: (((((COVID‐19) OR SARS‐CoV‐2) OR coronavirus) OR corona‐virus)) AND ((graft*) OR transplant*)
Scopus: (((((COVID‐19) OR SARS‐CoV‐2) OR coronavirus) OR corona‐virus)) AND ((graft*) OR transplant*)
PubMed: (((((COVID‐19) OR SARS‐CoV‐2) OR coronavirus) OR corona virus)) AND ((graft*) OR transplant*)
Moosavi SA, Mashhadiagha A, Motazedian N, Hashemazar A, Hoveidaei AH, Bolignano D. COVID‐19 clinical manifestations and treatment strategies among solid‐organ recipients: A systematic review of cases. Transpl Infect Dis. 2020;22:e13427. 10.1111/tid.13427
REFERENCES
- 1. Sahay M, Kute V, Prasad N. Corona, COVID and kidney transplantation. Indian J Transplant. 2020;14(1):1‐4. [Google Scholar]
- 2. Wong HYF, Lam HYS, Fong AH, et al. Frequency and distribution of chest radiographic findings in COVID‐19 positive patients. Radiology. 2019;296(2):E72‐E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ju CR, Lian QY, Zhang JH, et al. Recommended prophylactic and management strategies for severe acute respiratory syndrome coronavirus 2 infection in transplant recipients. Chronic Dis Translat Med. 2020;6(2):87‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halazun KJ, Rosenblatt R. Lest we forget. Am J Transplant. 2020;20(7):1785‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar D, Manuel O, Natori Y, et al. COVID‐19: a global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020;20(7):1773‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russell B, Moss C, George G, et al. Associations between immune‐suppressive and stimulating drugs and novel COVID‐19‐a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartiromo M, Borchi B, Botta A, et al. Threatening drug‐drug interaction in a kidney transplant patient with coronavirus disease 2019 (COVID‐19). Transplant Infect Dis. 2020. 10.1111/tid.13286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoveidaei AH, Soufi H, Dehghani SM, Imanieh MH. Successful treatment of idiopathic thrombocytopenic purpura after liver transplant: a case report. Exp Clin Transplant. 2018. 10.6002/ect.2018.0083 [DOI] [PubMed] [Google Scholar]
- 9. Saigal S, Gupta S, Sudhindran S, et al. Liver transplantation and COVID‐19 (Coronavirus) infection: guidelines of the liver transplant Society of India (LTSI). Hep Intl. 2020;14(4):429‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu H, He X, Wang Y, et al. Management of COVID‐19 in patients after liver transplantation: Beijing working party for liver transplantation. Hep Intl. 2020;14(4):432‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boyarsky BJ, Chiang TP, Werbel WA, et al. Early impact of COVID‐19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20(7):1809‐1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abrishami A, Samavat S, Behnam B, Arab‐Ahmadi M, Nafar M, Sanei TM. Clinical course, imaging features, and outcomes of COVID‐19 in kidney transplant recipients. Eur Urol. 2020;78(2):281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aigner C, Dittmer U, Kamler M, Collaud S, Taube C. COVID‐19 in a lung transplant recipient. J Heart Lung Transplant. 2020;39(6):610‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short‐term outcome of 20 kidney transplant patients admitted for SARS‐CoV2 pneumonia. Kidney Int. 2020;97(6):1083‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arpali E, Akyollu B, Yelken B, Tekin S, Turkmen A, Kocak B. Case report: a kidney transplant patient with mild COVID‐19. Transplant Infect Dis. 2020. 10.1111/tid.13296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID‐19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Billah M, Santeusanio A, Delaney V, Cravedi P, Farouk SS. A catabolic state in a kidney transplant recipient with COVID‐19. Transpl Int. 2020. 10.1111/tri.13635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bin L, Wang Y, Zhao Y, Shi H, Zeng F, Chen Z. Successful treatment of severe COVID‐19 pneumonia in a liver transplant recipient. Am J Transplant. 2020;20(7):1891‐1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bussalino E, De Maria A, Russo R, Paoletti E. Immunosuppressive therapy maintenance in a kidney transplant recipient SARS‐CoV‐2 pneumonia: a case report. Am J Transplant. 2020;20(7):1922‐1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen S, Yin Q, Shi H, et al. A familial cluster, including a kidney transplant recipient, of coronavirus disease 2019 (COVID‐19) in Wuhan, China. Am J Transplant. 2020;20(7):1869‐1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cozzi E, Faccioli E, Marinello S, et al. COVID‐19 pneumonia in lung transplant recipients: report of two cases. Am J Transplant. 2020. 10.1111/ajt.15993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Barros Machado DJ, Ianhez LE. COVID‐19 pneumonia in kidney transplant recipients‐ where we are? Transplant Infect Dis. 2020. 10.1111/tid.13306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Donato MF, Lampertico P, Rossi G. Health status of liver transplanted patients during the coronavirus outbreak in Italy: a large single center experience from milan. Clin Gastroenterol Hepatol. 2020. 10.1016/j.cgh.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farfour E, Picard C, Beaumont L, et al. COVID‐19 in lung‐transplanted and cystic fibrosis patients: be careful. J Cyst Fibros. 2020;19(3):e16‐e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernandez‐Ruiz M, Andres A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020;20(7):1849‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fontana F, Alfano G, Mori G, et al. Covid‐19 pneumonia in a kidney transplant recipient successfully treated with Tocilizumab and Hydroxychloroquine. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gandolfini I, Delsante M, Fiaccadori E, et al. COVID‐19 in kidney transplant recipients. Am J Transplant. 2020;20(7):1941‐1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao F, Zheng KI, Gu JY, George J, Zheng MH. COVID‐19 and liver transplantation: lessons learned from three reported cases. Transplant Infect Dis. 2020. 10.1111/tid.13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID‐19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20(7):1875‐1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hammami MB, Garibaldi B, Shah P, et al. Clinical course of COVID‐19 in a liver transplant recipient on hemodialysis and response to tocilizumab therapy: a case report. Am J Transplant. 2020. 10.1111/ajt.15985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holzhauser L, Lourenco L, Sarswat N, Kim G, Chung B, Nguyen AB. Early experience of COVID‐19 in two heart transplant recipients: case reports and review of treatment options. Am J Transplant. 2020. 10.1111/ajt.15982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsu JJ, Gaynor P, Kamath M, et al. COVID‐19 in a high‐risk dual heart and kidney transplant recipient. Am J Transplant. 2020;20(7):1911‐1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang J, Lin H, Wu Y, et al. COVID‐19 in post‐transplantation patients‐ report of two cases. Am J Transplantation. 2020;20(7):1879‐1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson KM, Belfer JJ, Peterson GR, Boelkins MR, Dumkow LE. Managing COVID‐19 in renal transplant recipients: a review of recent literature and case supporting corticosteroid‐sparing immunosuppression. Pharmacotherapy. 2020;40(6):517‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kates OS, Fisher CE, Stankiewicz‐Karita HC, et al. Earliest cases of coronavirus disease 2019 (COVID‐19) identified in solid organ transplant recipients in the United States. Am J Transplant. 2020;20(7):1885‐1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ketcham SW, Adie SK, Malliett A, et al. Coronavirus disease 2019 in heart transplant recipients in Southeastern Michigan ‐ case series. J Card Fail. 2020;26(6):457‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim Y, Kwon O, Paek JH, et al. Two distinct cases with COVID‐19 in kidney transplant recipients. Am J Transplant. 2020. 10.1111/ajt.15967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kocak B, Arpali E, Akyollu B, et al. A case report of oligosymptomatic kidney transplant patients with COVID‐19: do they pose a risk to other recipients? Transplant Proc. 2020. 10.1016/j.transproceed.2020.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koczulla RA, Sczepanski B, Koteczki A, et al. SARS‐CoV‐2 infection in two patients following recent lung transplantation. Am J Transplant. 2020. 10.1111/ajt.15998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Latif F, Clerkin KJ, et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. 2020. 10.1001/jamacardio.2020.2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marx D, Moulin B, Fafi‐Kremer S, et al. First case of COVID‐19 in a kidney transplant recipient treated with belatacept. Am J Transplant. 2020;20(7):1944‐1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mathies D, Rauschning D, Wagner U, et al. A Case of SARS‐CoV‐2‐pneumonia with successful antiviral therapy in a 77‐year‐old male with heart transplant. Am J Transplant. 2020;20(7):1925‐1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meziyerh S, Zwart TC, van Etten RW, et al. Severe COVID‐19 in a renal transplant recipient: a focus on pharmacokinetics. Am J Transplant. 2020;20(7):1896‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Montagud‐Marrahi E, Cofan F, Torregrosa JV, et al. Preliminary data on outcomes of SARS‐CoV‐2 infection in a Spanish single centre cohort of kidney recipients. Am J Transplant. 2020. 10.1111/ajt.15970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nair V, Jandovitz N, Hirsch JS, et al. COVID‐19 in kidney transplant recipients. Am J Transplant. 2020;20(7):1819‐1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Namazee N, Mahmoudi H, Afzal P, Ghaffari S. Novel corona virus 2019 pneumonia in a kidney transplant recipient. Am J Transplant. 2020. 10.1111/ajt.15999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US Epicenter. Am J Transplant. 2020;20(7):1800‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seminari E, Colaneri M, Sambo M, et al. SARS Cov2 infection in a renal transplanted patients. A case report. Am J Transplant. 2020;20(7):1882‐1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Travi G, Rossotti R, Merli M, et al. Clinical outcome in solid organ transplant recipients with COVID‐19: A single‐center experience. Am J Transplant. 2020. 10.1111/ajt.16069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tschopp J, L'Huillier AG, Mombelli M, et al. First experience of SARS‐CoV‐2 infections in solid organ transplant recipients in the Swiss transplant cohort study. Am J Transplant. 2020. 10.1111/ajt.16062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang J, Li X, Cao G, Wu X, Wang Z, Yan T. COVID‐19 in a kidney transplant patient. Eur Urol. 2020;77(6):769‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xuan TM, Wang XX, Pu XY, Han WL, Guo XG. Primary percutaneous coronary intervention in a COVID‐19 patient with ST‐segment elevation myocardial infarction after lung transplantation: a case report. J Zhejiang Univ Sci B. 2020;21(5):411‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang H, Chen Y, Yuan Q, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020;77(6):742‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang M, Zhang J, Shi H, Liu B, Zeng F. Viral shedding prolongation in a kidney transplant patient with COVID‐19 pneumonia. Am J Transplant. 2020. 10.1111/ajt.15996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhong Z, Zhang Q, Xia H, et al. Clinical characteristics and immunosuppressants management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20(7):1916‐1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu L, Gong N, Liu B, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77(6):748‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhu L, Xu X, Ma K, et al. Successful x of COVID‐19 pneumonia in a renal transplant recipient with long‐term immunosuppression. Am J Transplant. 2020;20(7):1859‐1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. AlGhamdi M, Mushtaq F, Awn N, Shalhoub S. MERS CoV infection in two renal transplant recipients: case report. Am J Transplant. 2015;15(4):1101‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe acute respiratory syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant. 2003;3(8):977‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutierrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sevarolli MDL, Loschi TM, Pereira E, Miyaura VTO, Baccan M, Pavao DN. Epidemiologic profile of transplant patients treated by the physiotherapy. Transplant Proc. 2018;50(3):831‐834. [DOI] [PubMed] [Google Scholar]
- 63. TRANSPLANTATION GOODA . Organ donation and transplantation activities executive summary 2017.
- 64. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Manuel O, Estabrook M. RNA respiratory viral infections in solid organ transplant recipients: guidelines from the American Society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9). 10.1111/ctr.13511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nam HH, Ison MG. Community‐acquired respiratory viruses in solid organ transplant. Curr Opin Organ Transplant. 2019;24(4):483‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduction and Targeted Therapy. 2020;5(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang L. C‐reactive protein levels in the early stage of COVID‐19. Med Mal Infect. 2020;50(4):332‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID‐19 and Multi‐Organ Response. Curr Probl Cardiol. 2020;45(8). 10.1016/j.cpcardiol.2020.100618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in‐hospital death of patients with COVID‐19. Kidney Int. 2020;97(5):829‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID‐19 pandemic: AASLD expert panel consensus statement. Hepatology (Baltimore, MD). 2020;72(1):287‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Clinical management of severe acute respiratory infection when COVID‐19 is suspected March 13, 2020. https://www.who.int/publications‐detail/clinical‐management‐of‐severe‐acute‐respiratory‐infection‐when‐novel‐coronavirus‐(ncov)‐infection‐is‐suspected. Accessed May 27, 2020.
- 76. Jawhara S. Could intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID‐19 and strengthen the immune system of new patients? Int J Mol Sci. 2020;21(7):2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID‐19). StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC.; 2020. [Google Scholar]
- 78. Cheng D, Wen J, Liu Z, Lv T, Chen J. Coronavirus disease 2019 in renal transplant recipients: report of two cases. Transpl Infect Dis. 2020. 10.1111/tid.13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li F, Cai J, Dong N. First cases of COVID‐19 in heart transplantation from China. J Heart Lung Transplant. 2020;39(5):496–497. 10.1016/j.healun.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ning L, Liu L, Li W, et al. Novel coronavirus (SARS‐CoV‐2) infection in a renal transplant recipient: case report. Am J Transplant. 2020;20(7):1864–1868. 10.1111/ajt.15897 [DOI] [PMC free article] [PubMed] [Google Scholar]


