Abstract
Coronavirus disease 2019 or COVID‐19 is an emerging viral disease caused by a member of the betacoronavirus family, SARS‐CoV‐2. Since its' emergence in December 2019, it has rapidly caused close to half a million fatalities globally. Data regarding the impact of COVID‐19 on pregnancy are limited. Here, we review pathological findings in placentas from women who tested positive for SARS‐CoV‐2 as well as information on pregnancy outcomes associated with related and highly pathogenic coronaviruses (ie, severe acute respiratory syndrome (SARS‐COV) and the Middle East respiratory syndrome, MERS). We present immune‐inflammatory correlates of COVID‐19 in pregnancy and review the role of the Renin Angiotensin System in the pathogenesis of COVID‐19 in pregnancy. Greater understanding of the pathogenesis of SARS‐CoV‐2 in the placenta will yield important insight into potential therapeutic interventions for pregnant women with COVID‐19.
Keywords: ACE2, AT1, COVID‐19, placenta, pre‐eclampsia, renin‐angiotensin system
1. INTRODUCTION
1.1. Severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) and COVID‐19
The novel coronavirus SARS‐CoV‐2 was first reported in December 2019 in the city of Wuhan, Hubei Province, China. Physicians noted cases of pneumonia of unknown origin, which were later characterized as being caused by a novel betacoronavirus. 1 Within a couple of months, SARS‐CoV‐2 has become a global emergency resulting in a wide spectrum of disease from asymptomatic infections to severe respiratory failure. The disease caused by SARS‐CoV‐2 has been termed COVID‐19. SARS‐CoV‐2 is an enveloped, positive sense, single‐stranded RNA (30 kb genome size) and made up of four structural proteins (spike surface glycoprotein, envelope protein, membrane protein, and nucleocapsid protein) and non‐structural proteins which are active targets of ongoing vaccine research. 2 , 3 SARS‐CoV‐2 shares more than 80% similarity with the SARS‐CoV 4 , 5 , 6 which caused an outbreak in 2002 and shares 50% sequence similarity with Middle East respiratory syndrome (MERS) coronavirus that caused an outbreak in 2012. 7 The mortality rate of SARS‐CoV‐2 is low compared with SARS and MERS; however, approximately 1% of infected people develop severe acute respiratory distress syndrome (ARDS) that requiring a critical level of care. While routes of SARS‐CoV‐2 transmission remain under active investigation, the predominant routes are currently thought to be through aerosols and fomites. 1 Although much of the world's focus is on the lethal respiratory effects of COVID‐19, especially in the elderly, emerging information suggests that COVID‐19 puts younger women and their babies at increased risk of pregnancy complications. For example, in the largest case series of COVID‐19 in pregnancy, nearly 50% of women infected with the responsible virus, SARS‐CoV2, delivered preterm, and infected women had higher rates of the hypertensive disorder pre‐eclampsia and preterm premature rupture of membranes than non‐infected women. 8 , 9 Whether this virus can be transmitted vertically during pregnancy remains under investigation; however, its presence in the placenta and breast milk has been documented in a few recent studies. 10 , 11 , 12 , 13 , 14 The mechanisms underlying the effects of COVID‐19 on pregnant women are also unknown and are under investigation.
Physiological changes that occur during pregnancy, including alterations in immunity, can increase susceptibility to viral infections. In addition, the immature innate and adaptive immune system of the developing fetus makes them highly susceptible to infections with potential deleterious consequences for fetal and neonatal brain development and function. Given the rapid spread of SARS‐CoV‐2 globally, it is important to understand the immediate and long‐term risks of the virus to the maternal‐infant dyad. In this review, we examine current evidence for the pathogenesis of SARS‐CoV‐2 infection in pregnancy and discuss possible mechanisms of disease progression in the context of pregnancy outcomes (Figure 1).
FIGURE 1.
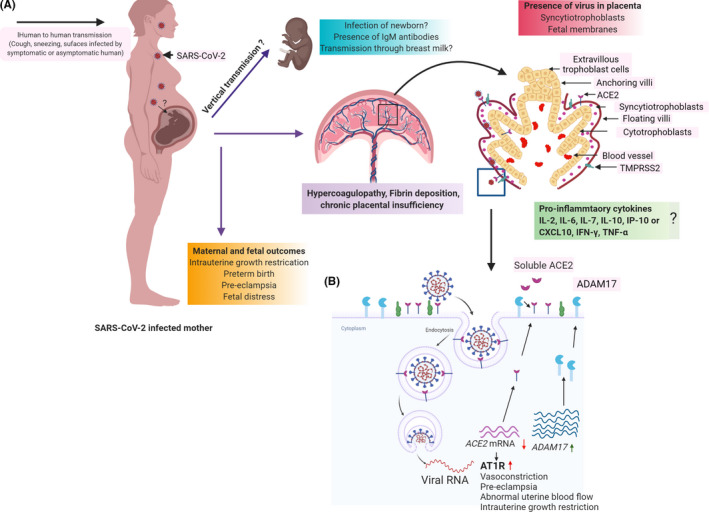
Schematic of SARS‐CoV‐2 mechanisms, targets, and outcomes in pregnancy
1.2. COVID‐19 and pregnancy
A number of case series have been published or posted on the preprint server MedArkiv or BioArkiv that describe clinical features and outcomes in pregnant women with COVID‐19. Overall, there is limited evidence of vertical transmission or significant mortality for pregnant women with COVID‐19. However, multiple adverse perinatal outcomes have been reported, including increased risk of miscarriage, premature rupture of membranes, preterm birth, and stillbirth, and pre‐eclampsia. 8 , 9 , 15 , 16 , 17 Below, we highlight four aspects of COVID‐19 in pregnancy: Vertical Transmission or not, immunological changes; placental pathology; and RAS pathway and pre‐eclampsia.
1.2.1. Vertical Transmission or not?
SARS‐CoV‐2 infected pregnant women are susceptible to pneumonia and preterm birth before <37 and 34 weeks of gestation and the risk of miscarriage early in pregnancy may be increased. 8 , 9 , 14 Liu et al, reported that in a cohort of 13 COVID‐19 infected pregnant women, nearly 50% (6/13) delivered prematurely. 15 Zeng et al, described cases of neonatal early‐onset infection in a cohort study of 33 pregnant women in Wuhan with confirmed positive SARS‐CoV‐2 and charted the infection status and clinical course of their neonates. Of the 33 neonates, 3 tested positive for SARS‐CoV‐2 and displayed symptoms of COVID‐19 (including fever, respiratory distress syndrome, or shortness of breath). All three neonates were delivered by cesarean section (C‐section) due to non‐reassuring fetal status. This study concluded that, given the stringent infection control and prevention procedures in place during these births, the 3 infants were likely to have acquired their infection from their mothers antenatally. 18 Another group published a case report of a neonate born to a mother with confirmed COVID‐19 who was delivered by C‐section in a negative pressure room and not held by its mother following birth. At 2 hours post‐birth, this infant tested positive for both SARS‐CoV‐2 IgG and IgM. Presence of IgM suggested maternal‐fetal transmission of the antibodies, but the infant did not develop any symptoms of COVID‐19. 19 A systemic and meta‐analysis review analyzed data from 17 studies including 2567 pregnancies identified the most common outcome of SARS‐CoV‐2 infection as iatrogenic preterm birth and C‐section deliveries. Perinatal death incidence was observed in less than 1% (12 stillbirth and 4 neonatal deaths), and 1%‐2% neonates were positive with SARS‐CoV‐2. 12 In another study, amniotic fluid and neonatal throat swab tested positive with SARS‐CoV‐2 and three neonates were also positive with SARS‐CoV‐2. 20
However, other studies have concluded no evidence of vertical transmission. For example, one study demonstrated that, in a cohort of 9 women who had confirmed COVID‐19 pneumonia, no infant tested positive for SARS‐CoV‐2 through amniotic fluid, cord blood, throat swab, or breast milk samples. 21 , 22 Another study with 38 SARS‐CoV‐2 infected pregnant women in their third trimester of pregnancy reported no maternal deaths or instances of vertical transmission to the new born after testing all neonatal samples and a few placentas for SARS‐CoV‐2. 23 Huntley et al, examined 13 studies comprising 538 pregnancies and reported neonatal death in 0.3% (1/313) of neonates and no vertical transmission. 24 Chen et al, reported a 20% incidence of preterm birth (out of 118 pregnancies), but no vertical transmission. 25 The preponderance of evidence thus far does not indicate a significant role for vertical transmission; but, it remains a global imperative to understand the impact of COVID‐19 on maternal morbidity and mortality.
1.2.2. Immunology of COVID‐19 in pregnancy
Pregnancy is an immunological condition in which the semi‐allogenic fetus grows in the mother's uterus. The immunological environment during pregnancy changes as pregnancy proceeds initially through Th1 (pro‐inflammatory response) during the first trimester, and then changes to Th2 (anti‐inflammatory response) by the second trimester, and again polarizes toward a Th1 phenotype at the end of third‐trimester concomitant with initiation of parturition. 26 Furthermore, the number of immune cells also vary during pregnancy with a higher prevalence of innate immune cells (NK cells, monocytes) than adaptive immune cells (fewer B and T cells). 26 SARS‐CoV‐2 infection appears to induce a reduction in the number of lymphocytes relative to inflammatory monocytes, which could induce a cytokine storm leading to ARDS and multi‐system organ failure. 27 , 28 In support of this hypothesis, plasma from severely infected COVID‐19 patients showed enhanced levels of interleukins (IL‐2, IL‐6, IL‐7, and IL‐10), granulocyte‐colony stimulating factor (GCSF), interferon‐γ‐inducible protein 10 (IP‐10 or CXCL10), monocyte chemoattractant protein 1 (MCP‐1), macrophage inflammatory protein 1 alpha (MIP‐1α), and tumor necrosis factor α (TNF‐α). 14 , 29 The high level of TNF‐α during early pregnancy is associated with defects in early embryo development 30 pregnancy‐related complications upon malaria infection. 31 A recent systematic review identified increased level of C‐reactive protein (CRP) in a majority of COVID‐19 positive pregnant women. 20 Taken together, the cytokine storm (pro‐inflammatory cytokines) induced by SARS‐CoV‐2 might place pregnant women at greater risk by causing an increased pro‐inflammatory state with the potential to impact fetal brain development; thus, increasing the risk for autism, mental health disorders or schizophrenia. 26 , 32 , 33 , 34
Currently, there are no studies reported on decidual immune cell interactions with SARS‐CoV‐2, but there are studies on the effect of SARS‐CoV‐2 on peripheral blood leukocytes. One key difference noted in peripheral blood leukocytes from those suffering severe COVID‐19 symptoms, versus those with mild symptoms, was a decrease in IFN‐γ and TNF‐α in CD4+ T cells from the severe symptoms group. In addition, increased levels of granzyme B and perforin have been noted in CD8+ T cells. 35 This study also found an increase in CD8+ T cell exhaustion in the severe group, which suggests that CD8+ T exhaustion may impair the patients ability to mount an appropriate immune response to SARS‐CoV‐2. 35 , 36 Lymphopenia associated with confirmed SARS‐CoV‐2 patients has also been widely reported. 37 It remains to be determined whether there is a change in decidual NK cells or T‐cell populations at the maternal‐fetal interface in COVID‐19 positive placentas.
1.2.3. Placental pathology in COVID‐19
The placenta is a critical organ which provides nutrition and protect the developing fetus from infections. 38 Histopathological examination of the placenta can provide crucial evidence of the underpinnings of clinically observed maternal and fetal outcomes. Placentas positive for SARS‐CoV‐2 has frequently shown increased deposition of fibrin and infiltration of monocytes and neutrophils in subchorial space. 16 A study comprising 11 SARS‐CoV‐2 positive pregnant women reported presence of SARS‐CoV‐2 in placental and fetal membrane swabs of 3 patients. 39 A case report of a woman who was infected with SARS‐CoV‐2 at 22 weeks gestation with severe hypertension, coagulopathy, and pre‐eclampsia showed that the placenta and umbilical cord were positive for SARS‐CoV‐2. Furthermore, localization of SARS‐CoV‐2 spike protein and RNA was found in the villi and perivillous fibrin and infiltration of macrophages was reported in placental sections. 10 A study by Shanes et al, reports placental pathological findings from 16 SARS‐CoV‐2 positive pregnant women (15 delivered‐ third trimester, 1 delivered in 2nd trimester due to intrauterine fetal demise) showing systemic inflammation and a hypercoagulated state. There was evidence of placental hyperfusion defects in maternal vessels and oxygenation in the intervillous space affecting perinatal outcome. 40 In all studies thus far, vascular villous lesions including fibrin deposition within and around the villi and infarcts have been reported, suggestive of pre‐eclampsia. This finding is similar to those noted from placentas from MERS and SARS‐CoV positive patients reporting acute and chronic placental insufficiency associated with intrauterine growth restriction (IUGR) or miscarriage in 40% of affected cases during pregnancy. 41 , 42 , 43 In case of MERS, placental abruption was observed due to inflammation in the mother which resulted in fetal demise and preterm delivery. 44 , 45 Furthermore, SARS‐CoVinfection has been shown to result in IUGR, small for gestational age neonates, preterm birth, and spontaneous miscarriage (mainly in first trimester). The fibrin deposition in subchorionic villi in 3/7 placentas has also been observed. 42 , 43 Together, these reports suggest that, based on histopathological findings, changes associated with fibrin deposition, immune filtration, and decidual vasculopathy have emerged as one of the most commonly observed pattern of SARS‐CoV‐2 infection at the level of the placenta. 8
1.2.4. RAS in pregnancy and COVID‐19
SARS‐CoV‐2 enters host cells by binding to the angiotensin‐converting enzyme 2, ACE2. In addition to ACE2, SARS‐COV‐2 requires the cellular protease, TMPRSS2, to cleave viral spike protein and facilitate fusion of viral and cellular membranes. 46 , 47 Single‐cell transcriptomic data from a first‐trimester placenta demonstrated that ACE2 is highly expressed on placental cells including extravillous trophoblasts, villous cytotrophoblasts and syncytiotrophoblasts (STBs), decidual stromal cells, and fetal endothelial cells. However, this is contested by a recent paper using single‐cell RNA sequence analyses of the placenta, and new single‐cell/nuclei RNA‐sequencing data, suggesting that there is negligible co‐expression of ACE2 and TMPRSS2 in trophoblasts as well as in third‐trimester chorioamniotic membranes. 48 , 49 TMPRSS2 has been reported to be expressed on all three trophoblast cell types. 50 Human Protein Atlas (https://www.proteinatlas.org/) indicates low level of expression of ACE2 and TMPRSS2 in trophoblasts and endothelial cells. The level of expression and possible role for ACE2 in COVID‐19 in the placenta remains to be elucidated.
Upon entry of SARS‐CoV‐2, the levels of ACE2 are decreased as the protein is targeted for degradation via the clathrin‐mediated endocytic pathway leading to reduced availability of ACE2 on the surface. 47 , 51 Along with ACE2, SARS‐CoV2 affects other components of the Renin Angiotensin System (RAS) such as ADAM metallopeptidase domain 17 (ADAM17) that can cleave membrane‐bound ACE2 receptors and downregulate its expression. 52 The loss of ACE2 has also been observed in patients clinically experiencing hypertension, diabetes, cardiac arrest, or other comorbid conditions 53 (Figure 1B). RAS, an endocrine system plays a critical regulatory role in fetal‐placental circulation, facilitating adequate placental blood flow for fetal oxygenation and maturation as early as 6 weeks of gestation. 54 All of the components of RAS are present in the placenta (villous and extravillous) and contribute to placental function including invasion, migration, and angiogenesis. 55 Expression of ACE2 and the cofactor TMPRSS2 increases as pregnancy progresses. 56 Dysregulation of RAS is associated with adverse pregnancy outcomes, particularly pre‐eclampsia and fetal growth restriction. 57 Additionally, placental trophoblasts and fetal endothelial cells are sensitive to changes in RAS activity. 55 , 58 Several animal models have been employed to show the impact of RAS on the uteroplacental blood flow during pregnancy. 59 , 60 Mice lacking ace2 exhibit placental dysfunction as evidenced by abnormal uterine remodeling, placental hypoxia, and uterine artery reactivity to vasoconstrictors. 61
A possible mechanism of impact of SARS‐CoV‐2 infection in pregnancy could be due to disruption of the RAS pathway due to sequestration and degradation of ACE2 by virus binding. Placental trophoblasts are particularly responsive to changes in angiotensin receptor concentrations that occur during pregnancy, and some women with pre‐eclampsia harbor an autoantibody that stimulates the Angiotensin 1 (AT1) receptor. 62 This AT1 autoantibody binds to AT1 receptors on trophoblasts where it synergistically acts with angiotensin 2 to impair placentation, and thereby normal RAS function, which is implicated in pre‐eclampsia. Indeed, the circulating levels of renin and angiotensin 1 are lower in women with pre‐eclampsia than in normotensive women. 58 A possible link to pre‐eclampsia due to COVID‐19 is supported by the placental findings of fibrin deposition and coagulopathy. It remains to be determined whether SARS‐CoV‐2 infection results in adverse pregnancy outcomes due to disruption of the RAS system, which is actively under investigation by us and other groups.
2. SUMMARY
Emerging information suggests that COVID‐19 puts women and their babies at increased risk of pregnancy complications like pre‐eclampsia, preterm birth. The entry of SARS‐CoV‐2 is mediated by ACE2, and its expression increases during pregnancy which may provide favorable conditions for SARS‐CoV‐2 infection. The presence of virus in placental villi and fetal membranes suggests that the virus can access the placenta and could affect fetal development. Vertical transmission of SARS‐CoV‐2 appears to be limited but is not yet completely ruled out. In any case, the cytokine storm induced by SARS‐CoV‐2 could result in increased morbidity and mortality among pregnant women with the potential to adversely affect the developing fetus and neonate, even in the absence of vertical viral transmission. Severity of illness, timing of infection, and other underlying conditions during pregnancy could also impact the adverse effects of COVID‐19. Formulation of better strategies, models, and targets need to be identified to decrease the impact of viral infection to improve maternal and fetal outcomes.
3. GAPS
More studies are needed to better understand whether SARS‐CoV‐2 can be vertically transmitted.
The effect of SARS‐CoV‐2 during early pregnancy (1st and 2nd trimester) on maternal/infant health.
The direct (vertical transmission) or indirect effect (via placental insufficiency) or inflammatory milieu due to SARS CoV‐2 infection on the developing fetus.
The effect of SARS‐CoV‐2 postnatally in the newborn.
Neonatal impact of SARS‐CoV‐2 infected mother breastfeeding.
The mechanism of SARS‐CoV‐2 infection, entry in placenta, role of immune cells.
4. DISCLOSURES
The authors have no financial conflict disclosures. IUM serves on the Scientific Advisory Board of Luca Biologics.
ACKNOWLEDGMENTS
This work was funded in part from grants from NIH/NICHD R01HD091218 (to IUM); McDonnell International Scholars Academy (to IUM); and support from WUSM ICTS NIH CTSA Grant Number UL1TR002345 (to EBC). Given the emergence of numerous preprints and rapid publication pace on COVID‐19 in pregnancy, we regret if we missed any recent studies.
Verma S, Carter EB, Mysorekar IU. SARS‐CoV2 and pregnancy: An invisible enemy?. Am J Reprod Immunol. 2020;84:e13308. 10.1111/aji.13308
Contributor Information
Sonam Verma, Email: sonam.verma@wustl.edu.
Indira U. Mysorekar, Email: imysorekar@wustl.edu.
REFERENCES
- 1. Pedersen SF, Ho Y‐C. SARS‐CoV‐2: a storm is raging. J Clin Invest. 2020;130(5):2202‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thanh Le T, Andreadakis Z, Kumar A, et al. The COVID‐19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:305‐306. 10.1038/d41573-020-00073-5 [DOI] [PubMed] [Google Scholar]
- 3. Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID‐19 coronavirus (SARS‐CoV‐2) based on SARS‐CoV immunological studies. Viruses. 2020;12:254. 10.3390/v12030254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu J, Zhao S, Teng T, et al. Systematic comparison of two animal‐to‐human transmitted human coronaviruses: SARS‐CoV‐2 and SARS‐CoV. Viruses. 2020;12:244. 10.3390/v12020244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gralinski LE, Menachery VD. Return of the coronavirus: 2019‐nCoV. Viruses. 2020;12:135. 10.3390/v12020135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, Li X, Li T, et al. The genetic sequence, origin, and diagnosis of SARS‐CoV‐2. Eur J Clin Microbiol Infect Dis. 2020. 10.1007/s10096-020-03899-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Mascio D, Khalil A, Saccone G, et al. Outcome of Coronavirus spectrum infections (SARS, MERS, COVID 1–19) during pregnancy: a systematic review and meta‐analysis. Am J Obstet Gynecol MFM. 2020;2:100107. 10.1016/j.ajogmf.2020.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID‐19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99(7):823‐829. 10.1111/aogs.13867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hosier H, Farhadian SF, Morotti RA, et al. SARS‐CoV‐2 infection of the placenta. J Clin Invest. 2020. 10.1172/JCI139569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Algarroba GN, Rekawek P, Vahanian SA, et al. Visualization of SARS‐CoV‐2 virus invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020. 10.1016/j.ajog.2020.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khalil A, Kalafat E, Benlioglu C, et al. SARS‐CoV‐2 infection in pregnancy: a systematic review and meta‐analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;100446 10.1016/j.eclinm.2020.100446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bastug A, Hanifehnezhad A, Tayman C, et al. Virolactia in an asymptomatic mother with COVID‐19. Breastfeed Med. 2020. 10.1089/bfm.2020.0161 [DOI] [PubMed] [Google Scholar]
- 14. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Chen H, Tang K, Guo Y. Clinical manifestations and outcome of SARS‐CoV‐2 infection during pregnancy. J Infect. 2020. 10.1016/j.jinf.2020.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baud D, Greub G, Favre G, et al. Second‐trimester miscarriage in a pregnant woman with SARS‐CoV‐2 infection. JAMA. 2020;323(21):2198. 10.1001/jama.2020.7233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu H, Wang L‐L, Zhao S‐J, et al. Why are pregnant women susceptible to COVID‐19? An immunological viewpoint. J Reprod Immunol. 2020;139:103122. 10.1016/j.jri.2020.103122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeng L, Xia S, Yuan W, et al. Neonatal early‐onset infection with SARS‐CoV‐2 in 33 neonates born to mothers with COVID‐19 in Wuhan, China. JAMA Pediatr. 2020;174(7):722–725. 10.1001/jamapediatrics.2020.0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dong L, Tian J, He S, et al. Possible vertical transmission of SARS‐CoV‐2 from an infected mother to her newborn. JAMA. 2020;323(18):1846–1848. 10.1001/jama.2020.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Juan J, Gil MM, Rong Z, et al. Effect of coronavirus disease 2019 (COVID‐19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56:15‐27. 10.1002/uog.22088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815. 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly JC, Dombrowksi M, O'neil‐Callahan M, Kernberg AS, Frolova AI, Stout MJ. False‐negative COVID‐19 testing: considerations in obstetrical care. Am J Obstet Gynecol MFM. 2020;100130. 10.1016/j.ajogmf.2020.100130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwartz DA. An analysis of 38 pregnant women with COVID‐19, Their newborn infants, and maternal‐fetal transmission of SARS‐CoV‐2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020;144(7):799–805. 10.5858/arpa.2020-0901-SA [DOI] [PubMed] [Google Scholar]
- 24. Huntley BJF, Huntley ES, Di Mascio D, et al. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐Co‐V‐2) infection: A systematic review. Obstet Gynecol. 2020. 10.1097/AOG.0000000000004010 [DOI] [PubMed] [Google Scholar]
- 25. Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with Covid‐19 in Wuhan, China. N Engl J Med. 2020;382:e100. 10.1056/NEJMc2009226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17:469‐482. 10.1038/nri.2017.64 [DOI] [PubMed] [Google Scholar]
- 27. Villar J, Zhang H, Slutsky AS. Lung repair and regeneration in ARDS: Role of PECAM1 and Wnt signaling. Chest. 2019;155:587‐594. 10.1016/j.chest.2018.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529‐539. 10.1007/s00281-017-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tetro JA. Is COVID‐19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22:72‐73. 10.1016/j.micinf.2020.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. 2018;49:397‐412. 10.1016/j.immuni.2018.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fried M, Kurtis JD, Swihart B, et al. Systemic inflammatory response to malaria during pregnancy is associated with pregnancy loss and preterm delivery. Clin Infect Dis. 2017;65:1729‐1735. 10.1093/cid/cix623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chudnovets A, Lei J, Na Q, et al. Dose‐dependent structural and immunological changes in the placenta and fetal brain in response to systemic inflammation during pregnancy. Am J Reprod Immunol, 2020:e13248. 10.1111/aji.13248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. al‐Haddad BJS, Oler E, Armistead B, et al. The fetal origins of mental illness. Am J Obstet Gynecol. 2019;221:549‐562. 10.1016/j.ajog.2019.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J Dev Neurosci. 2005;23:299‐305. 10.1016/j.ijdevneu.2004.05.005 [DOI] [PubMed] [Google Scholar]
- 35. Zheng H‐Y, Zhang MI, Yang C‐X, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID‐19 patients. Cell Mol Immunol. 2020;17(5):541‐543. 10.1038/s41423-020-0401-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;221(11):1762‐1769. 10.1093/infdis/jiaa150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heerema‐McKenney A. Defense and infection of the human placenta. APMIS. 2018;126:570‐588. 10.1111/apm.12847 [DOI] [PubMed] [Google Scholar]
- 39. Penfield CA, et al. Detection of SARS‐COV‐2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM. 2020;100133. 10.1016/j.ajogmf.2020.100133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID‐19. Am J Clin Pathol. 2020;154:23‐32. 10.1093/ajcp/aqaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Favre G, Pomar L, Musso D, Baud D. 2019‐nCoV epidemic: what about pregnancies? Lancet. 2020;395:e40. 10.1016/S0140-6736(20)30311-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wong SF, Chow KM, Leung TN, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292‐297. 10.1016/j.ajog.2003.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ng WF, Wong SF, Lam A, et al. The placentas of patients with severe acute respiratory syndrome: a pathophysiological evaluation. Pathology. 2006;38:210‐218. 10.1080/00313020600696280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alfaraj SH, Al‐Tawfiq JA, Memish ZA. Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect. 2019;52:501‐503. 10.1016/j.jmii.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jeong SY, Sung SI, Sung J‐H, et al. MERS‐CoV infection in a pregnant woman in Korea. J Korean Med Sci. 2017;32:1717‐1720. 10.3346/jkms.2017.32.10.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pique‐Regi R, Romero R, Tarca AL, et al. Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife. 2019;8:e52004. 10.7554/eLife.52004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pique‐Regi R, Romero R, Tarca AL, et al. Does the human placenta express the canonical cell entry mediators for SARS‐CoV‐2? eLife 2020;9:e58716. 10.1101/2020.05.18.101485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rajagopalan S, Long EO. Cell atlas reveals the landscape of early pregnancy. Nature. 2018;563:337‐338. 10.1038/d41586-018-07317-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peron JPS, Nakaya H. Susceptibility of the elderly to SARS‐CoV‐2 infection: ACE‐2 overexpression, shedding, and antibody‐dependent enhancement (ADE). Clinics (Sao Paulo). 2020;75:e1912. 10.6061/clinics/2020/e1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lambert DW, Yarski M, Warner FJ, et al. Tumor necrosis factor‐alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe‐acute respiratory syndrome‐coronavirus (SARS‐CoV) receptor, angiotensin‐converting enzyme‐2 (ACE2). J Biol Chem. 2005;280:30113‐30119. 10.1074/jbc.M505111200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang K, Gheblawi M, Oudit GY. Angiotensin converting enzyme 2: a double‐edged sword. Circulation. 2020. 10.1161/CIRCULATIONAHA.120.047049 [DOI] [PubMed] [Google Scholar]
- 54. Irani RA, Xia Y. The functional role of the renin‐angiotensin system in pregnancy and preeclampsia. Placenta. 2008;29:763‐771. 10.1016/j.placenta.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pringle KG, Tadros MA, Callister RJ, Lumbers ER. The expression and localization of the human placental prorenin/renin‐angiotensin system throughout pregnancy: roles in trophoblast invasion and angiogenesis? Placenta. 2011;32:956‐962. 10.1016/j.placenta.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 56. Pavličev M, Wagner GP, Chavan AR, et al. Single‐cell transcriptomics of the human placenta: inferring the cell communication network of the maternal‐fetal interface. Genome Res. 2017;27:349‐361. 10.1101/gr.207597.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856‐2869. 10.1161/CIRCULATIONAHA.109.853127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Valdés G, Neves L, Anton L, et al. Distribution of angiotensin‐(1–7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27:200‐207. 10.1016/j.placenta.2005.02.015 [DOI] [PubMed] [Google Scholar]
- 59. Ito M, Itakura A, Ohno Y, et al. Possible activation of the renin‐angiotensin system in the feto‐placental unit in preeclampsia. J Clin Endocrinol Metab. 2002;87:1871‐1878. 10.1210/jcem.87.4.8422 [DOI] [PubMed] [Google Scholar]
- 60. Moritz KM, Cuffe J, Wilson LB, et al. Review: sex specific programming: a critical role for the renal renin‐angiotensin system. Placenta. 2010;31(Suppl):S40‐46. 10.1016/j.placenta.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 61. Yamaleyeva LM, Pulgar VM, Lindsey SH, et al. Uterine artery dysfunction in pregnant ACE2 knockout mice is associated with placental hypoxia and reduced umbilical blood flow velocity. Am J Physiol Endocrinol Metab. 2015;309:E84‐94. 10.1152/ajpendo.00596.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945‐952. 10.1172/JCI4106 [DOI] [PMC free article] [PubMed] [Google Scholar]


