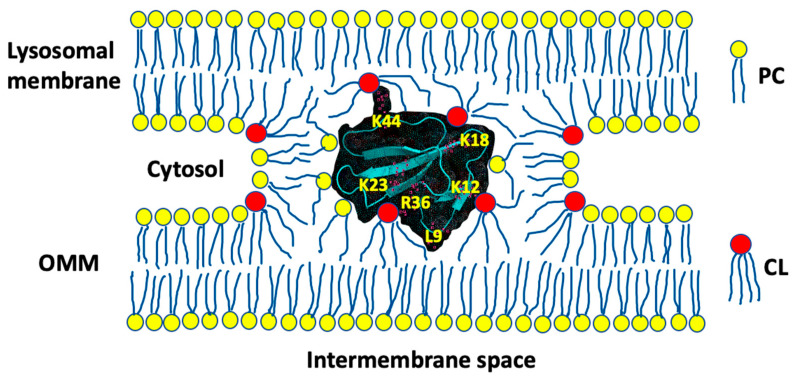
Figure 12.
Intermembrane junction with a cardiotoxin in its center. Cardiotoxin CTII is given in a cartoon and dots representation. Amino acid residues R36 and L9 are predicted by our MD data as the key residues in the initial binding of CTII to the OMM. R36 binds to CL of the OMM while L9 penetrates to the hydrophobic region of the outer leaflet of the OMM. K5, K12, K18, K35, (not shown in this figure as they are on another side of CTII) and K44 presumably attract anionic phospholipids of neighboring lysosomes. K12 is also predicted by our AutoDock data along with K18, K23 and some other residues, which are not shown in this figure, to establish short-range intermolecular bonds with polar groups of CL and PC to support the further embedding of CTII into the OMM.