Figure 1.
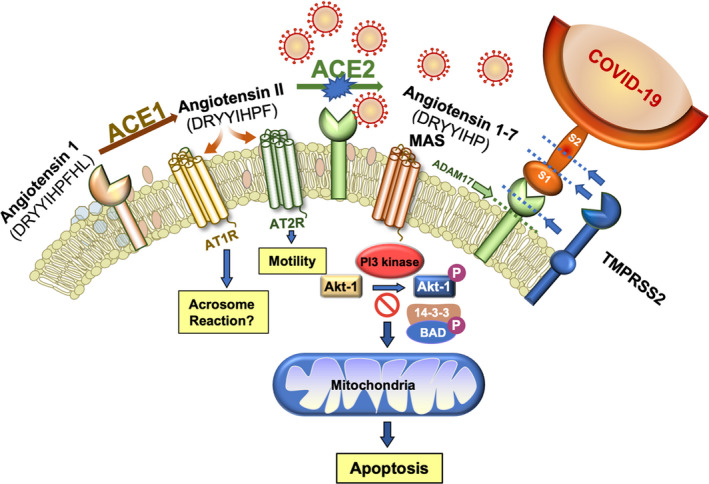
The angiotensin system plays a critical role in the survival and functionality of human spermatozoa but also creates a vulnerability to COVID‐19 attack. Angiotensin 1 is a biologically inactive decapeptide that is cleaved by ACE1 to create angiotensin II, which in turn activates the AT1R and AG2R receptors, both of which are present in these cells. Angiotensin II is further processed by ACE2 to generate angiotensin 1‐7 which binds the MAS receptor activating PI3K. The latter then phosphorylates AKT, which maintains cell viability by phosphorylating key regulators of sperm apoptosis such as BAD. As long as BAD is phosphorylated, it is held in abeyance by a 14‐3‐3 keeper protein. However, if the PI3/AKT pathway becomes compromised, BAD dephosphorylates, is released from its association with 14‐3‐3, and moves to the mitochondria where it inactivates anti‐apoptotic factors and promotes the intrinsic apoptotic cascade. The spike protein on COVID‐19 specifically targets ACE2 and in so doing removes an important stimulus for PI3K/AKT, thereby compromising sperm viability. Subsequent to COVID‐19 binding, the ectodomain of ACE2 may be removed by ADAM proteases and shed from the sperm surface. Alternatively proteases from the TMPRSS‐family, either as intrinsic components of the sperm plasma membrane or delivered by seminal prostasomes, can facilitate fusion between the virus and the sperm surface by cleaving ACE2 and the viral spike proteins (S1 and S2) at the sites indicated by dashed lines, thereby completing the transformation of this cell from procreating gamete to viral vector
