Abstract
Treatment options for severe acute respiratory syndrome‐related coronavirus‐2 (SARS‐CoV‐2) are limited with no clarity on efficacy and safety profiles. We performed a systematic review and meta‐analysis of studies on patients ≥18 years reporting data on therapeutic interventions in SARS‐CoV‐2. Primary outcome was all‐cause mortality and secondary outcomes were rates of mechanical ventilation, viral clearance, adverse events, discharge, and progression to severe disease. Pooled rates and odds ratios (OR) were calculated. Twenty‐nine studies with 5207 patients were included. Pooled all‐cause mortality in intervention arm was 12.8% (95% confidence interval [CI]: 8.1%‐17.4%). Mortality was significantly higher for studies using hydroxychloroquine (HCQ) for intervention (OR: 1.36; 95% CI: 0.97‐1.89). Adverse events were also higher in HCQ subgroup (OR: 3.88; 95% CI: 1.60‐9.45). There was no difference in other secondary outcomes. There is a need for well‐designed randomized clinical trials for further investigation of every therapeutic intervention for further insight into different therapeutic options.
Keywords: COVID‐19, HCQ, hydroxychloroquine, meta‐analysis, SARS‐CoV‐2
Highlights
Our meta‐analysis of 5207 patients showed that the use of hydroxychloroquine was associated with increased mortality and adverse event rates in Severe acute respiratory syndrome‐related coronavirus‐2 infection and other therapeutic interventions did not show any difference in outcomes.
Abbreviations
- CI
confidence interval
- COVID‐19
coronavirus disease 2019
- FDA
Food and Drug Administration
- HCQ
hydroxychloroquine
- IQR
interquartile range
- NIH
National Institute of Health
- NNH
number needed to harm
- NNT
number needed to treat
- OR
odds ratio
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- RCT
randomized controlled trials
- SARS‐CoV‐2
severe acute respiratory syndrome‐related coronavirus‐2
- WHO
World Health Organization
1. INTRODUCTION
Severe acute respiratory syndrome‐related coronavirus‐2 (SARS‐CoV‐2) is the 7th virus of the coronavirus family known to infect humans. 1 By March, the World Health Organization (WHO) had declared SARS‐CoV‐2 as a pandemic, the third pandemic in the 21st century after the SARS outbreak in 2003 and H1N1 influenza in 2009. SARS‐CoV‐2 tends to cause a plethora of symptoms with fever, cough, myalgia, fatigue, loss of taste, appetite, and diarrhea to name a few. It is also known to affect multiple organ systems leading to acute respiratory distress syndrome, encephalitis, myocarditis, hepatitis, acute kidney injury, and hypercoagulable state leading to stroke and pulmonary embolism. The COVID‐19 disease caused by SARS‐CoV‐2 can be classified as mild, moderate, severe, and critical disease based on clinical, imaging and laboratory parameters. 2 The natural history of the disease is such that most patients typically have mild disease with spontaneous resolution of symptoms by 10 to 14 days needing symptomatic management and home self‐quarantine. Elderly population, as well as patients with medical comorbidities, are at higher risk of developing moderate to severe disease. 3 As per the Chinese Center for Disease Control and Prevention data in a cohort of 72 314 patients, clinical deterioration tends to typically occur in the second week of onset of symptoms with need for hospitalization and close monitoring in 14% of patients and around 5% of patients require invasive ventilation. 4 Several therapeutic interventions like Hydroxychloroquine (HCQ), Chloroquine, Remdesivir, Corticosteroids, Tocilizumab, and convalescent plasma therapy have been attempted, but currently, there is no known intervention that has reduced mortality in COVID‐19 patients. These questions bring into focus the need of a comprehensive systematic review of the published literature to collate the available evidence. The aim of this systematic review and meta‐analysis is to assess if any intervention provides mortality benefit, other clinically relevant outcomes, and also ascertain the safety profile.
2. METHODS
This systematic review was performed as per the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses recommendations. 5 The protocol is provided as Appendix 1. Institutional review board approval was not required for this study since no patient identifiers were disclosed.
2.1. Data sources
A systematic electronic search was performed in PubMed/MEDLINE, Embase, Cochrane Central, Google Scholar, MedRxiv databases to identify published and prepublished studies reporting outcomes related to interventions for SARS‐CoV‐2 infection, from 1 December 2019 to 11 May 2020. The Medical Subject Heading/Entree terms is provided in Appendix 2. An independent review of the abstracts and full paper articles was done (VT and BV). The duplicates were removed and the titles of articles were evaluated. The full‐length papers of the shortlisted articles were assessed for the eligibility criteria. The articles that fulfilled the inclusion criteria were shortlisted for final systematic review. The included study references were cross‐searched for additional studies. The articles were reviewed independently by two authors (VT and BV) and any disagreement was resolved by consensus with a third author (MR). Reasons for excluding studies were documented.
2.2. Eligibility criteria
The inclusion criteria were as follows: (a) studies reporting outcomes for treatment in SARS‐CoV‐2 infection; (b) all studies including randomized controlled trials (RCTs), prospective, retrospective, and case series; (c) full‐length studies; (d) patients more than 18 years of age. Exclusion criteria were: (a) preclinical studies, epidemiological, and descriptive studies without intervention for SARS‐CoV‐2 patients; (b) abstracts.
2.3. Data extraction and quality assessment
The data were extracted by two authors independently into predefined forms. The following data were extracted from the studies: first author, mean age, study design, number of patients, gender, rates of: mortality, clinical improvement, mechanical ventilation, progression to severe disease, viral clearance, discharge, and adverse events. Data for both intervention and control arms (for available studies) were extracted separately. Quality assessment was performed only for RCTs as most of the other studies were retrospective in nature with short hospital courses for duration of treatment. Cochrane risk bias tool was used for study quality assessment for RCTs. 6
2.4. Definitions and outcomes
The definitions of outcomes that were assessed are provided in Appendix 3. The intervention arm consisted of patients receiving the drug or the therapeutic intervention while the control arm patients received standard of care treatment for SARS‐CoV‐2 without a specific intervention. The primary outcomes were the all‐cause mortality in the intervention arm and in comparison, with control arm. The secondary outcomes were rates of clinical recovery, need for mechanical ventilation, viral clearance, radiological improvement, discharge, and adverse events in intervention arm and comparison with control arm. Median duration for viral clearance and clinical recovery was also calculated from available studies. Number needed to treat (NNT) and number needed to harm (NNH) were defined as the number of patients who needed to be treated to provide benefit or harm in at least one patient, comparing intervention and control arms for respective outcomes.
2.5. Statistical analysis
Percentages for categorical variables and median with interquartile range (IQR) for continuous variables were presented. Differences in medians were calculated using the Mann‐Whitney‐Wilcoxon test. Proportions with pooled rates with 95% confidence intervals (CIs) were calculated for individual arms. Odds ratios (OR) comparing with control arm were reported with 95% CI and P value of less than .05 was considered statistically significant. Random effects model described by DerSimonian and Laird was used for analysis. Corresponding forest plots were constructed for both primary and secondary outcomes. NNT and NNH were calculated using the inverse of the differences in benefit or harm between the intervention and control arms for the respective outcomes. Study heterogeneity was assessed using Inconsistency index (I 2 statistic) with low, moderate, substantial, and considerable heterogeneity indicated by I 2 value of 0% to 30%, 31% to 60%, 61% to 75%, and 76% to 100%, respectively. All analyses were performed using statistical softwares Open Meta analyst (CEBM, Brown University, RI) and Review Manager Version 5.3 (The Nordic Cochrane Center, Copenhagen, Denmark). Subgroup analyses were performed for the following, when data were available and also to address heterogeneity in primary outcome if present: (a) intervention specific; (b) disease severity specific; (c) RCTs only.
3. RESULTS
3.1. Study search and study characteristics
The literature search resulted in 3664 articles, of which 65 articles underwent full review and 29 were included in the final analysis (Figure 1). 3 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 Among included studies, 19 were performed in China, four in France, four in the United States, one in Brazil, and one in South Korea. Eight studies were RCTs, four were prospective studies and the remaining 17 were retrospective studies. Fifteen studies were published and the remaining were prepublished. Seventeen studies had a drug or intervention being tested with a control group for comparison. For intervention, 12 studies used HCQ based treatment (two studies had azithromycin along with HCQ in same arm, three had azithromycin in separate arm, and one study was comparison of HCQ with Lopinavir/Ritonavir), five studies used antiviral agents (two studies with Lopinavir/Ritonavir, one with Baloxavir/Marboxil, and Favipravir and two with Remdesivir), two were Tocilizumab based single‐arm studies, five used corticosteroids (three with control arm) and five studies were single‐arm plasma therapy based. There were 3624 patients in the intervention arm (mean age: 55.9 ± 8.4 years, 62% males) and 1583 patients (mean age: 52.5 ± 8.5 years, 60.7% males) in the control arm. The median duration of follow‐up was 14 days (IQR: 9‐24.5) and the range was 6 to 32 days across all studies. The demographics and study characteristics have been provided in Table 1 .
Figure 1.
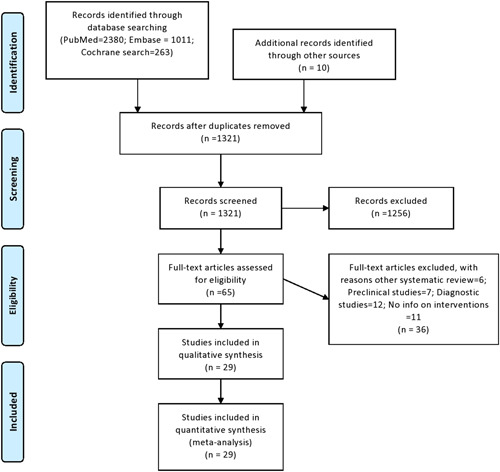
PRISMA Flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
Table 1.
Study design, demographics, intervention arms, and outcomes
| Study | Country | Design | Arms | Groups | Number of of patients | Mean age | Males | Primary outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I1 | I2 | C | I1 | I2 | C | I1 | I2 | C | ||||||
| Chen et al 14 | China | RCT | 2 | HCQ vs control | 31 | 31 | 44.1 | 45.2 | 14 | 15 | Clinical recovery | |||
| Chen et al 15 | China | RCT | 2 | HCQ vs control | 15 | 15 | 50.5 | 46.7 | 9 | 12 | Viral clearance | |||
| Borba et al 16 | Brazil | RCT | 2 | HCQ low dose vs high dose | 41 | 40 | 54.7 | 47.4 | 10 | 10 | Adverse events | |||
| Magagnoli et al 13 | USA | Retrospective | 3 | HCQ vs HCQ + AZ vs control | 97 | 113 | 158 | 70 | 68 | 69 | 97 | 113 | 158 | Mortality, intubation |
| Molina et al 12 | France | Case series | 1 | HCQ high dose + AZ | 11 | 7 | Viral clearance | |||||||
| Mahevas et al 11 | France | Retrospective | 2 | HCQ vs control | 84 | 97 | 59 | 62 | 65 | 63 | ICU, mortality | |||
| Gautret et al 8 | France | Prospective | 3 | HCQ vs HCQ + AZ vs control | 14 | 6 | 16 | 51.2 | 37.3 | 9 | 6 | Viral clearance | ||
| Gautret et al 9 | France | Prospective | 1 | HCQ + AZ | 80 | 52.5 | 43 | Clinical recovery, viral clearance, length of stay | ||||||
| Tang et al 7 | China | Open label | 2 | HCQ vs control | 75 | 75 | 48 | 46.1 | 42 | 40 | Viral clearance | |||
| Huang et al 32 | China | RCT | 2 | HCQ vs L/R | 10 | 12 | 41.5 | 53 | 7 | 6 | Viral clearance, imaging recover, LOS | |||
| Geleris et al 10 | USA | Retrospective | 2 | HCQ vs control | 811 | 565 | 474 | 307 | Intubation, death | |||||
| Rosenberg et al 34 | USA | Retrospective | 4 | HCQ + AZ vs HCQ vs AZ vs control | 735 | 271 | 221 | 61.4 | 65.5 | 64 | 456 | 158 | 110 | All‐cause mortality, cardiac arrest and ECG abnormalities |
| Cao et al 26 | China | RCT | 2 | L/R vs control | 99 | 100 | 58 | 58 | 61 | 59 | Time to clinical recovery | |||
| Li Y et al 27 | China | RCT | 3 | L/R vs arbidol vs control | 34 | 35 | 17 | 50.7 | 50.5 | 44.3 | 17 | 16 | 7 | Viral clearance |
| Lou et al 28 | China | RCT | 3 | B/M vs Favipravir vs control | 10 | 9 | 10 | 53.5 | 58 | 46.6 | 7 | 7 | 7 | Viral clearance |
| Grein et al 23 | USA | Prospective | 1 | Remdesivir | 53 | 64 | 40 | Clinical course | ||||||
| Wang et al 19 | China | RCT | 2 | Remdesivir vs Placebo | 158 | 78 | 66 | 64 | 89 | 51 | Time to clinical recovery | |||
| Luo et al 21 | China | Retrospective | 1 | Tocilizumab | 15 | 73 | 12 | Laboratory improvement | ||||||
| Xu X et al 20 | China | Retrospective | 1 | Tocilizumab | 21 | 56.8 | 18 | Clinical course | ||||||
| Fang et al 29 | China | Retrospective | 2 | Oral steroids vs control | 9 | 46 | 40.2 | 39.9 | 5 | 22 | Viral clearance | |||
| Fang et al 29 | China | Retrospective | 2 | Intravenous steroids vs control | 16 | 7 | 60.6 | 54.3 | 12 | 5 | Viral clearance | |||
| Guan et al 3 | China | Retrospective | 1 | steroids | 204 | ICU, intubation, mortality | ||||||||
| Lu et al 25 | China | Retrospective | 2 | Steroids vs control | 151 | 93 | 64 | 59 | 83 | 45 | Mortality | |||
| Lu et al 25 | China | Retrospective | 2 | Steroid vs control | 31 | 31 | 57 | 58 | 16 | 16 | Mortality | |||
| Wu et al 4 , 18 | China | Retrospective | 2 | Steroids vs control | 50 | 34 | Mortality | |||||||
| Wang et al 19 | China | Retrospective | 2 | Intravenous steroids vs control | 26 | 20 | 54 | 53 | 16 | 10 | Clinical course | |||
| Zhang et al 17 | China | Case series | 1 | Plasma | 4 | 2 | ⋯ | |||||||
| Ahn et al 33 | Korea | Case series | 1 | Plasma | 2 | 69 | 1 | ⋯ | ||||||
| Shen et al 31 | China | Case series | 1 | Plasma | 5 | 3 | Clinical recovery | |||||||
| Duan et al 30 | China | Retrospective | 1 | Plasma | 10 | 52.5 | 6 | Adverse events | ||||||
| Ye et al24 | China | case series | 1 | Plasma | 6 | 64 | 3 | Clinical recovery | ||||||
Abbreviations: AZ, azithromycin; B/M, baloxavir/marboxil; ECG, electrocardiogram; HCQ, hydroxychloroquine; I1, intervention 1; I2, intervention 2; ICU, intensive care unit; L/R, lopinavir/ritonavir; USA, United States of America.
3.2. Risk of bias assessment
Eight RCTs were part of this meta‐analysis. Of these three were at low risk of bias and five were at high risk. Risk of bias summary has been provided in Appendix 4.
3.3. Primary outcome: all‐cause mortality
Twenty‐four studies provided data on mortality in the intervention arm and the pooled all‐cause in‐hospital mortality rate was 12.8% (95% CI: 8.1%‐17.4%) for a median follow‐up duration of 14 (IQR: 10‐18.5) days (Table 2). Comparing the mortality between the intervention arm and control arms, 10 studies (n = 3894) provided the data, with a pooled rate of 17.1% (95% CI: 9.1%‐27.4%) in the intervention arm and 14.8% (95% CI: 9.4%‐20.1%) in the control arm, with no significant difference between the two groups (OR: 1.36; 95% CI: 0.97‐1.89; I 2 = 46%; P = .07) (Figure 2A). The NNH was calculated to be 43. When analysis was restricted to only four HCQ based studies (n = 3152), the mortality was significantly higher in the HCQ group (OR: 1.86; 95% CI: 1.38‐2.50; I 2 = 29%; P < .001) (NNH—13). (Figure 2B) A further subgroup analysis for only two studies (n = 212) which used only HCQ for treatment without any other confounders like azithromycin and the mortality was still significantly higher in the HCQ group (OR: 2.17; 95% CI: 1.26‐3.72; I 2 = 43%) (NNH—9). Comparing intervention and control arms, subgroup analysis performed for antiviral studies only (n = 550) (OR: 0.83; 95% CI: 0.49‐1.38), steroid‐based studies (n = 192) (OR: 0.96; 95% CI: 0.40‐2.31), moderate to severe disease patients (n = 2184) (OR: 1.09; 95% CI: 0.56‐1.57), severe disease patients (n = 627) (OR: 0.87; 95% CI: 0.58‐1.31) (Appendix Figure 1) and RCTs only (n = 550) (OR: 0.83; 95% CI: 0.49‐1.38) (Appendix Figure 2), did not show a statistically significant difference between the two groups (Appendix Table 1).
Table 2.
Study outcomes in the intervention and control groups with odds ratios
| Outcome | Intervention group only | Intervention group studies common with control group | Control group only | Odds ratio |
|---|---|---|---|---|
| All‐cause mortality | 11.9% (95% CI: 7.7%‐16.1%) (n = 2071) | 16.2% (95% CI: 8.8%‐23.6%) (n = 1557) | 15.4% (95% CI: 9.1%‐21.6%) (n = 1110) | 1.22 (95% CI: 0.85‐1.75; I 2 = 39%) (n = 2667) |
| Mechanical ventilation rate | 18.6% (95% CI: 10.9%‐26.3%) (n = 1456) | 13.5% (95% CI: 7%‐19.9%) (n = 1382) | 9.8% (95% CI: 4.5%‐ 15.2%) (n = 935) | 1.58 (95% CI: 0.60‐4.15; I 2 = 85%) (n = 2317) |
| Antiviral clearance rate | 80% (95% CI: 70.7%‐89.4%) (n = 393) | 74.9% (95% CI: 59.5%‐90.3%) (n = 257) | 66.8% (95% CI: 42.6%‐91.1%) (n = 204) | 1.86 (95% CI: 0.76‐4.54; I 2 = 58% (n = 461) |
| Clinical recovery rate | 79.7% (95% CI: 78.9%‐ 88.4%) (n = 558) | 64.1% (95% CI: 51.5%‐76.8%) (n = 340) | 56.9% (95% CI: 43.8%‐69.9%) (n = 243) | 1.41 (95% CI: 0.99‐2.02, I 2 = 0%) (n = 583) |
| Progression to severe disease | 11.6% (95% CI: 5.4%‐17.8%) (n = 387) | 13.4% (95% CI: 2.2%‐24.6%) (n = 218) | 12.8 (95% CI: 4.8%‐ 20.8%) (n = 168) | 1.19 (95% CI: 0.67‐2.13; I 2 = 0%) (n = 386) |
| Radiological improvement rate | 86.3% (95% CI: 77.2%‐95.5%) (n = 140) | 83.6% (95% CI: 67.6%‐99.7%) (n = 107) | 82.6% (95% CI: 60.3%‐100%) (n = 60) | 1.02 (95% CI: 0.13‐8.07; I 2 = 63%) (n = 167) |
| Discharge rate | 69.8% (95% CI: 60.3%‐79.3%) (n = 1374) | 68.6% (95% CI: 62.2%‐75%) (n = 1171) | 78.6% (95% CI: 67.8%‐89.4%) (n = 800) | 0.55 (95% CI: 0.29‐1.03; I 2 = 81%) |
| Adverse events rate | 23.3% (95% CI: 12.1%‐34.5%) (n = 791) | 34% (95% CI: 13.9%‐54.1%) (n = 436) | 29.5% (95% CI: 9.4%‐49.6%) (n = 310) | 1.44 (95% CI: 0.70‐2.94; I 2 = 62%) (n = 754) |
Figure 2.
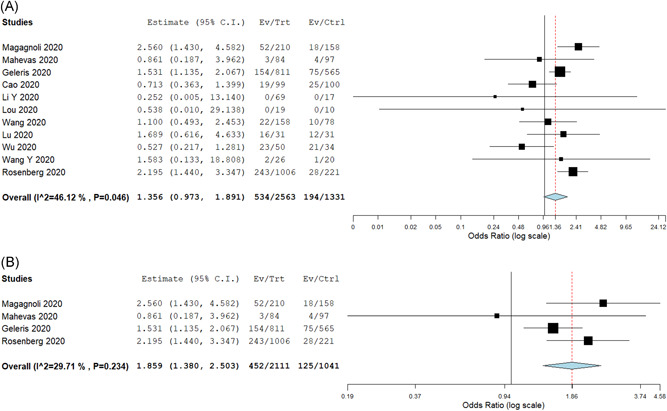
A, Odds ratio comparing all‐cause in hospital mortality in intervention and control arms. B, Odds ratio comparing all‐cause in hospital mortality in intervention and control arms in hydroxychloroquine based studies
3.4. Secondary outcomes
3.4.1. Rate of mechanical ventilation
Nine studies (n = 1456) reported need for mechanical ventilation in patients in the intervention arm, with a pooled intubation rate of 18.6% (95% CI: 10.9%‐26.3%) (Table 2). Comparing the seven studies (n = 2317) which also provided information on control population, the pooled rates in the intervention and control arms were 13.5% vs 9.8%, respectively, with no significant difference between the two groups (OR: 1.58; 95% CI: 0.60‐4.15; I 2 = 85%) (NNT—27) (Figure 3A). There was no significant difference in the outcome when analysis was restricted to HCQ and antiviral based studies.
Figure 3.
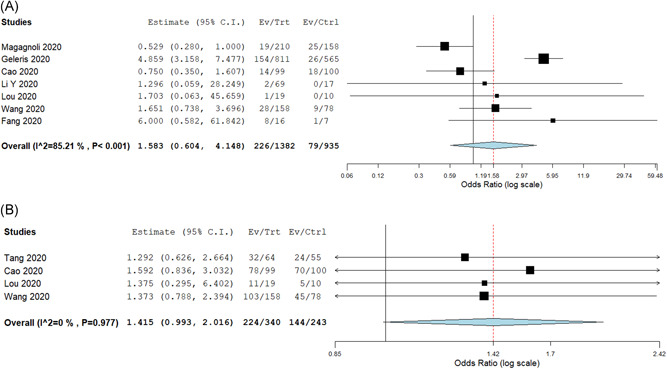
A, Odds ratio comparing rates of mechanical ventilation in intervention and control arms. B, Odds ratio comparing clinical recovery rates in intervention and control arms
3.4.2. Viral clearance
Fifteen studies reported data on either the proportion of patients with antiviral clearance at the end of the study or the median duration for antiviral clearance. The pooled proportion of patients with antiviral clearance in the intervention arm (n = 393) was 80% (95% CI: 70.7%‐89.4%). Comparing the six studies (n = 461) reporting data on antiviral clearance in intervention and control groups, the pooled rates were 74.9% vs 66.8%, respectively, with no significant difference between the two groups (OR: 1.86; 95% CI: 0.76‐4.54; I 2 = 58%) (NNT—10) (Appendix Figure 3). When the analysis was restricted to HCQ based and antiviral based studies, there was still no significant difference between the two groups. The median duration for antiviral clearance in the intervention arm (n = 308) was 6.1 (IQR: 4.3‐8.8) days and in the control arm (n = 170) was 9 (IQR: 4.5‐14) days, with no significant difference between the two groups (P = .37).
3.4.3. Clinical recovery
Fourteen studies reported data on either the proportion of patients who had clinical recovery or median time to clinical recovery. The pooled rate of proportion of patients with clinical recovery in the intervention arm (n = 558) was 79.7% (95% CI: 78.9%‐88.4%). Comparing the four studies reporting data in intervention and control arms, the pooled rates were 64.1% and 52.8% (NNT—9), respectively with no significant difference between the two groups (OR: 1.41; 95% CI: 0.99‐2.02; I 2 = 0%) (Figure 3B). Decrease in oxygen requirements in both groups was reported in two studies (n = 375), with no significant difference between both the groups (OR: 1.05; 95% CI: 0.65‐1.71; I 2 = 3%).
The median time to clinical recovery was 14 (IQR: 8.2‐19) days in the intervention group (n = 451) and 16 (IQR: 14.3‐22) days in the control group (n = 263), with no significant difference between the two groups (P = .25).
3.4.4. Progression to severe disease
Nine studies reported data on worsening of clinical status in the hospital in mild‐moderate severity patients, with a pooled rate of 11.6% (95% CI: 5.4%‐17.8%) in the intervention arm (n = 387) over a median duration of 13 (IQR: 9.5‐19.5) days. Comparing the pooled rates in five studies reporting the outcome in both groups (n = 386), the pooled rates were 13.4% and 12.8% in the intervention and control groups, respectively, with no significant difference between the two groups (OR: 1.19; 95% CI: 0.67‐2.13, I 2 = 0%). Subgroup analysis restricted to HCQ based and antiviral studies also did not reveal any significant difference.
3.4.5. Adverse events
Sixteen studies (n = 791) reported the rate of adverse events in the intervention group with a pooled adverse event rate of 23.3% (95% CI: 12.1%‐34.5%). Six studies (n = 754) compared intervention and control groups with pooled adverse event rates of 34% and 29.5%, respectively, with no significant difference between the two groups (OR: 1.44; 95% CI: 0.70‐2.94) (NNT—22) (Figure 4A). On subgroup analysis, the incidence of adverse events was significantly higher in the HCQ group (OR: 3.88; 95% CI: 1.60‐9.45; I 2 = 0%) (Figure 4B) with an NNH of 7, but there was no significant difference for studies with antiviral agents.
Figure 4.
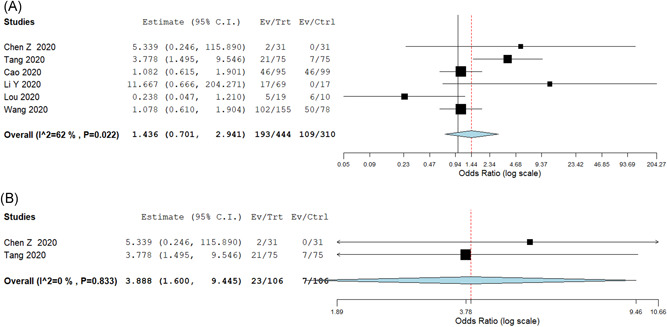
A, Odds ratio comparing adverse events rates in intervention and control arms. B, Odds ratio comparing adverse events in intervention and control arms in HCQ based studies. HCQ, hydroxychloroquine
There was also no significant difference between both the groups in radiological improvement and discharge rates. The individual pooled rates and ORs are provided in Table 2. Subgroup analysis for only RCTs for available outcomes is provided in Appendix Table 2.
4. DISCUSSION
In this systematic review and meta‐analysis of 5207 patients from 29 studies, pooled outcome for any therapeutic intervention including HCQ, Remdesivir, Lopinavir/Ritonavir, Steroids, Tocilizumab, Convalescent plasma therapy did not show a survival benefit compared to control arms. HCQ use was associated with significantly increased all‐cause inpatient mortality and adverse event rates. There were no significant benefits with any therapeutic intervention in changing the natural history of SARS‐CoV‐2 infection assessed in terms of rate of mechanical ventilation, viral clearance, time to discharge, time to clinical recovery, and radiological improvement.
HCQ increases the endosomal pH and prevents fusion of the host membrane with SARS‐CoV‐2 thereby interfering with the viral replication cycle. Several preclinical studies showed in vitro activity against SARS‐CoV‐2 leading to clinical use of HCQ in COVID‐19 disease. 35 , 36 An initial case series of 26 patients from France comparing HCQ and control groups suggested that HCQ leads to rapid antiviral clearance in 70% of patients compared to 12.5% in controls. This study was fraught with methodological inconsistencies like enrollment of asymptomatic individuals, omission of six patients from analysis (HCQ patients of whom one died and three were transferred to intensive care unit). 9 In a randomized study of 62 patients from China, patients treated with HCQ showed radiological improvement in resolution of lung lesions as well as reduction in clinical progression of disease. The commonality of the initial studies on HCQ was a relatively small sample size, inappropriate control groups, lack of clarity in defining the study outcomes. 14 Two larger prospective RCTs from France and Brazil show that HCQ/chloroquine use is associated with increased incidence of cardiac events with no survival benefit. 12 , 16 In view of conflicting data outcomes, the National Institute of Health (NIH), United States recommends that there is insufficient clinical data to recommend either for or against using chloroquine or HCQ for the treatment of COVID‐19. 37 There have also been reports of severe drug reaction with generalized exanthematous pustulosis reported with HCQ use. 38 Despite these issues, HCQ is currently one of the most commonly used medications in various parts of the world. Our analysis shows that HCQ based regimens had increased rate of mortality (NNH—13) and adverse events (NNH—7) compared to control patients. We hope our meta‐analysis adds more evidence to dampen its use in view of lack of benefit and increased side effects.
Remdesivir was originally designed for use against Ebola. Remdesivir was studied in severe COVID‐19 in China showed that the drug did not show any benefit in terms of time to recovery as well as 28‐day mortality outcome, though the study was terminated prematurely in view of difficulty in patient accrual. 22 ACTT NIH study showed that Remdesivir accelerated the time to recovery to 11 days compared to 15 days in the placebo arm with no mortality benefit. This prompted an emergency Food and Drug Administration authorization for use in COVID‐19 patients. There are calls for Remdesivir to be taken as the standard of care control in future clinical studies. There have been questions raised about the NIH study due to limitations such as change in the primary endpoint of study after initiation of the trial, lack of mortality benefit; study in moderate disease patients who tend to recover spontaneously by the end of second week. 39 There are ethical concerns among the scientific community that drugs without proven mortality benefit or reduction in the need for ventilatory support may be promoted in view of aggressive pharmaceutical lobbying. The current pandemic rings echo bells of the 2009 H1N1 pandemic and the desperate stockpiling of oseltamivir, whose proclaimed efficacy was claimed to be a byproduct of concealed information and aggressive lobbying by pharmaceutical companies. 40 The published or preprint data for other drugs like Favipravir, Baloxavir/Marboxil, corticosteroids, convalescent plasma are currently insufficient to make any specific recommendation and our meta‐analysis also suggests the same.
Our meta‐analysis shows that none of the so far studied interventions have a tangible benefit to change the course of disease outcomes with the current published evidence. The clinical studies that compare various interventions like the WHO sponsored solidarity trial that compares Remdesivir, chloroquine, or HCQ, lopinavir plus ritonavir, and interferon‐beta with control arm and has all‐cause mortality as the primary outcome is the need of the hour and the results are eagerly awaited. 41
The strengths of our study are as follows: we included 29 studies with more than 5200 patients in our analysis with various interventions. Our review is extensive, by including the available interventions and providing clinically relevant outcomes in comparison with controls. Several subgroup analyses were also performed based on study interventions and design. Heterogeneity in most of our outcomes was mild to moderate but we performed subgroup analysis in RCTs to further reduce the heterogeneity.
4.1. Limitations
Our study has several limitations. The study design, patient population, and the outcomes assessed were variable in different studies. Even though the intervention arms were clearly defined in most studies, some of the patients in those arms also received other medications and outcomes for such patients could not be excluded separately, which could have confounded the results. Different levels of disease severity of patients on study entry could lead to heterogeneity in outcomes but we tried to address it by performing subgroup analyses based on disease severity for outcomes when possible. Duration of follow‐up was variable across studies and entire patient data at the end of study may not have been represented which is a limitation of the published literature. Adverse events reported include medication‐related adverse events and also symptoms in both groups, which could be related to SARS‐CoV‐2, but this was unanimously reported across all studies. The dose of medications, especially HCQ, was variable in studies and dose based analysis could not be performed. Data from prepublished studies were also included in our analysis but we had included them to provide a more comprehensive overview to prevent misinterpretation of results to the best of our capabilities. The results of our study should hence be interpreted with caution keeping these limitations in mind.
5. CONCLUSIONS
In this meta‐analysis, there was no overall mortality or clinical benefit for most therapeutic interventions but the use of HCQ was associated with increased mortality rates and increased risk of adverse events in SARS‐CoV‐2 patients. None of the other therapeutic interventions like Lopinavir/Ritonavir, Remdesivir, Tocilizumab seemed to alter the natural clinical course of the disease based on the available literature. There is a need for well‐designed randomized clinical trials to further investigate the efficacy and safety of various therapeutic interventions.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
VTC: conceptualization of the study, data extraction, statistical analysis, drafting of the manuscript, final approval of the manuscript. BV: conceptualization of the study, data extraction, drafting of the manuscript, final approval of the manuscript. HKP: data extraction, critical revision of the manuscript, final approval of the manuscript. MS: Data extraction, critical revision of the manuscript, final approval of the manuscript. JM: critical revision of the manuscript, final approval of the manuscript. MR: conceptualization of the study, critical revision of the manuscript, final approval of the manuscript.
Supporting information
Supporting information
Thoguluva Chandrasekar V, Venkatesalu B, Patel HK, Spadaccini M, Manteuffel J, Ramesh M. Systematic review and meta‐analysis of effectiveness of treatment options against SARS‐CoV‐2 infection. J Med Virol. 2021;93:775–785. 10.1002/jmv.26302
Article summary line:
The use of hydroxychloroquine was associated with increased mortality and adverse event rates in Severe acute respiratory syndrome‐related coronavirus‐2 infection and other therapeutic interventions did not show any difference in outcomes.
REFERENCES
- 1. The species Severe acute respiratory syndrome‐related coronavirus: classifying . ‐nCoV and naming it SARS‐CoV‐2. Nature microbiology. 2020;5(4):536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate COVID‐19 [published online ahead of print April 24, 2020]. The N Engl J Med. 10.1056/NEJMcp2009249 [DOI] [PubMed]
- 3. Guan W‐j, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. The N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID‐19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients mainly with mild to moderate COVID‐19: an open‐label, randomized, controlled trial. BMJ. 2020;369. 10.1136/bmj.m1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gautret P, Lagier JC, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID‐19 patients with at least a six‐day follow up: a pilot observational study [published online ahead of print April 11, 2020]. Travel Med Infect Dis. 34:101663. 10.1016/j.tmaid.2020.101663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial [published online ahead of print March 20, 2020]. Int J Antimicro Ag. 105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed]
- 10. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with covid‐19. The N Engl J Med. 2020;382:2411‐2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahevas M, Tran V‐T, Roumier M, et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID‐19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. medRxiv. 2020. 10.1101/2020.04.10.20060699 [DOI]
- 12. Molina JM, Delaugerre C, Le Goff J, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID‐19 infection. Med Mal Infect. 2020;50:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid‐19. 2020. 10.1101/2020.04.16.20065920 [DOI] [PMC free article] [PubMed]
- 14. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID‐19: results of a randomized clinical trial. 2020. 10.1101/2020.03.22.20040758 [DOI]
- 15. Chen J, Li D, Ping L, et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID‐19. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):215‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection: a randomized clinical trial. JAMA Network Open. 2020;3(4):e208857. [DOI] [PubMed] [Google Scholar]
- 17. Zhang B, Liu S, Tan T, et al. Treatment with convalescent plasma for critically Ill patients with SARS Q1‐CoV‐2 infection. Chest. 2020;158:e9‐e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934‐943. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Jiang W, He Q, et al. Early, low‐dose and short‐term application of corticosteroid treatment in patients with severe COVID‐19 pneumonia: single‐center experience from Wuhan, China. medRxiv. 2020. 10.1101/2020.03.06.20032342 [DOI] [Google Scholar]
- 20. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970‐10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID‐19: A single center experience. J Med Virol. 2020;92(7):814‐818. 10.1056/NEJMcp2009249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395:1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe covid‐19. The N Engl J Med. 2020;382:2327‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID‐19 patients in Wuhan, China [published online ahead of print April 15, 2020]. J Med Virol. 10.1002/jmv.25882 [DOI] [PMC free article] [PubMed]
- 25. Lu X, Chen T, Wang Y, et al. Adjuvant corticosteroid therapy for critically ill patients with COVID‐19. Crit Care. 2020. 10.1101/2020.04.07.20056390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe covid‐19. N Engl J Med. 2020;382(19):1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y, Xie Z, Lin W, et al. An exploratory randomized controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID‐19 (ELACOI). medRxiv. 2020. 10.1101/2020.03.19.20038984 [DOI] [Google Scholar]
- 28. Lou Y, Liu L, Qiu Y. Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID‐19 Patients: an Exploratory Randomized, Controlled Trial. medRxiv. 2020. 10.1101/2020.04.29.20085761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fang X, Mei Q, Yang T, et al. Low‐dose corticosteroid therapy does not delay viral clearance in patients with COVID‐19. J Infect. 2020;81:147‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duan K, Liu B, Li C, et al. The feasibility of convalescent plasma therapy in severe COVID‐19 patients: a pilot study. medRxiv. 2020. 10.1101/2020.03.16.20036145 [DOI] [Google Scholar]
- 31. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically Ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang M, Tang T, Pang P, et al. Treating COVID‐19 with chloroquine. J Mol Cell Biol. 2020;12:322‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ahn JY, Sohn Y, Lee SH, et al. Use of convalescent plasma therapy in two COVID‐19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35(14):e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in‐hospital mortality in patients with COVID‐19 in New York State. JAMA. 2020;323(24):2493‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV‐2 infection in vitro. Cell Discov. 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. COVID‐19 Treatment Guidelines Panel Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines National Institutes of Health Available at https://wwwcovid19treatmentguidelinesnihgov/. Accessed May 14, 2020. [PubMed]
- 38. Schwartz R, Janniger C. Generalized pustular figurate erythema: a newly delineated severe cutaneous drug reaction linked with hydroxychloroquine. Dermatol Ther. 2020;33(3):e13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. NIH clinical trial shows Remdesivir accelerates recovery from advanced COVID‐19 . 2020. https://wwwnihgov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19
- 40. Belluz J. Tug of war for antiviral drugs data. BMJ. 2014;348:g2227. [DOI] [PubMed] [Google Scholar]
- 41.I. Public health emergency SOLIDARITY trial of treatments for COVID‐19 infection in hospitalized patients. BMC. 2020. 10.1186/ISRCTN83971151 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


