Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the cause of the novel coronavirus disease 2019 (COVID‐19), a highly pathogenic and sometimes fatal respiratory disease responsible for the current 2020 global pandemic. Presently, there remains no effective vaccine or efficient treatment strategies against COVID‐19. Non‐steroidal anti‐inflammatory drugs (NSAIDs) are medicines very widely used to alleviate fever, pain, and inflammation (common symptoms of COVID‐19 patients) through effectively blocking production of prostaglandins (PGs) via inhibition of cyclooxyganase enzymes. PGs can exert either proinflammatory or anti‐inflammatory effects depending on the inflammatory scenario. In this review, we survey the potential roles that NSAIDs and PGs may play during SARS‐CoV‐2 infection and the development and progression of COVID‐19.
Linked Articles
This article is part of a themed issue on The Pharmacology of COVID‐19. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.21/issuetoc
Keywords: COVID‐19, cytokine storm, inflammation, NSAID, prostaglandin, SARS‐CoV‐2
Abbreviations
- AA
arachidonic acid
- ACE2
angiotensin‐converting enzyme 2
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- AT1
type‐I pneumocyte
- AT2
type‐II pneumocyte
- BAL
bronchoalveolar lavage
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CoV
coronavirus
- COVID‐19
coronavirus disease 2019
- COX
cyclooxygenase
- DC
dendritic cell
- IAV
influenza A virus
- IL
interleukin
- IRF
IFN regulatory factor
- ISG
IFN‐stimulated genes
- MERS
Middle East respiratory syndrome
- mPGES
microsomal PGE synthase
- NETs
neutrophil extracellular traps
- NLRP3
NOD‐, LRR‐, and pyrin domain‐containing protein 3
- NSAID
non‐steroidal anti‐inflammatory drug
- Nsp
non‐structural proteins
- OR
odds ratio
- ORF
open reading frame
- PG
prostaglandin
- PGE2
prostaglandin E2
- PTGES
PGE synthase
- RSV
respiratory syncytial virus
- SAA
serum amyloid A
- SARS‐CoV‐1
severe acute respiratory syndrome coronavirus 1
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- Th1
type 1 helper T cell
- Th17
type 17 helper T cell
- TLR
toll‐like receptor
- TMPRSS2
transmembrane protease serine 2
- Treg
regulatory T cell
1. INTRODUCTION
The novel coronavirus disease 2019 (COVID‐19) was first reported in late 2019, Wuhan, China, and is now a global pandemic with >12 million confirmed cases and more than half a million deaths in over 200 countries and territories encompassing Africa, Americas, Eastern Mediterranean, Europe, South East Asia, and the Western Pacific (World Health Organization, COVID‐19 Situation Report 175, July 13, 2020). COVID‐19 is characterised by cough, fever, and shortness of breath confirmed by a study of 20,133 hospitalised UK patients (Docherty et al., 2020). COVID‐19 causes a spectrum of respiratory conditions differing in severity, including mild upper respiratory tract complications, acute respiratory distress syndrome (ARDS), and pneumonia (due to secondary infection) in more deleterious cases. Evidence from meta and genetic analyses indicates that co‐morbidities including chronic cardiac/kidney disease, non‐asthmatic chronic pulmonary disease, chronic obstructive pulmonary disease (COPD), cardiovascular disease, diabetes, liver disease, obesity, hypertension, and cerebrovascular disease are associated with higher incidence of mortality in COVID‐19 patients (Docherty et al., 2020; Wang, Li, Lu, & Huang, 2020). Furthermore, post‐mortem examinations reveal that tissue inflammation and organ dysfunction do not map to tissue/cellular dispersal of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) during fatal COVID‐19 (Dorward et al., 2020). Thus it was concluded that immune‐mediated as opposed to pathogen‐mediated organ inflammation and injury contributed to death in COVID‐19 patients (Dorward et al., 2020).
COVID‐19 is caused by SARS‐CoV‐2, which generally has a 4‐ to 5‐day average incubation period (prior to onset of symptoms), with 97.5% of symptomatic patients experiencing symptoms within 11.5 days (Lauer et al., 2020), although patients can also be asymptomatic. SARS‐CoV‐2 is normally transmitted via respiratory droplets to facial mucosal membranes (eyes, nose, and mouth) with high efficacy over short distances. SARS‐CoV‐2 belongs to a family of viruses named Coronaviridae, which are a broad range of single‐stranded, positive‐sense, RNA viruses that can cause respiratory, enteric, neurological, and hepatic diseases in multiple animal species and humans (Zumla, Chan, Azhar, Hui, & Yuen, 2016). α‐Coronaviruses (αCoVs, e.g., HCoV‐229E and HCoV‐NL63) and β‐coronaviruses (βCoVs, e.g., HCoV‐OC43 and HCoV‐HKU1) are two of four Coronaviridae genres which commonly infect humans (Liu, Liang, & Fung, 2020). These HCoVs usually cause mild to moderate upper respiratory tract infections, for example, the common cold.
However, over the last 18 years, three novel βCoVs have emerged causing three separate deadly respiratory disease outbreaks in humans. These novel βCoVs include severe acute respiratory syndrome coronavirus 1 (SARS‐CoV‐1) first identified in 2003, Guangdong, China; Middle East respiratory syndrome coronavirus (MERS‐CoV) first identified in 2012, Jeddah, Saudi Arabia; and most recently, SARS‐CoV‐2 (N. Chen, Zhou, et al., 2020; Khan et al., 2020). SARS‐CoV‐2 has caused far greater infectivity and transmission potential compared to SARS‐CoV‐1 and MERS‐CoV. SARS‐CoV‐2 shares roughly 79% and 50% genetic similarity with SARS‐CoV‐1 and MERS‐CoV, respectively (Lu et al., 2020), but is strikingly 96.1% genetically similar to bat CoV‐RaTG13 (H. Zhou, Chen, et al., 2020). This relatively high genetic similarity with SARS‐CoV‐1 suggests that SARS‐CoV‐2 may exert its pathogenesis via similar mechanisms.
Pathophysiology of SARS‐CoV‐2 culminates in airway injury (possibly by direct effects on epithelial cells) and powerful host inflammatory responses (e.g., neutrophilic and macrophage inflammation and cytokine storm), as previously observed in SARS‐CoV‐1 (G. Chen, Wu, et al., 2020; Huang et al., 2020). Hence, both viral infection and host responses contribute to disease severity. Regarding epidemiology, the correlation between increasing severity with age of infected SARS‐CoV‐2 patients does correspond with epidemiology of SARS‐CoV‐1 and MERS‐CoV (Tay, Poh, Rénia, MacAry, & Ng, 2020). SARS‐CoV‐1 infects various lung cells including ciliated airway epithelial cells, vascular endothelial cells, macrophages, and type II pneumocytes (AT2 cells) via binding to the host cell receptor, angiotensin‐converting enzyme 2 (ACE2), and the transmembrane protease serine 2 (TMPRSS2) for S protein priming (Cui, Li, & Shi, 2019; Hamming et al., 2004; Jia et al., 2005; Qian et al., 2013; H. Xu, Zhong, et al., 2020). Conversely, MERS‐CoV infects unciliated airway epithelial cells as well as type I (AT1 cells) and AT2 cells, via dipeptidyl peptidase‐4 (DPP‐4; CD26) (Cui et al., 2019; de Wit et al., 2013; Raj et al., 2013). As the infection route of SARS‐CoV‐2 is also via the ACE2 receptor and TMPRSS2, it is expected that lung cells are infected as defined in SARS‐CoV‐1 (Hoffmann et al., 2020; Walls et al., 2020; Zhao et al., 2020; P. Zhou, Yang, et al., 2020). Moreover, as ACE2 expression in lung cells is lowered during SARS‐CoV‐1 infection and loss of ACE2 function has been shown to contribute to acute lung injury (ALI) (Imai, Kuba, & Penninger, 2008; Imai et al., 2005; Kuba et al., 2005; Kuba, Imai, Rao, Jiang, & Penninger, 2006), a reduction in ACE2 receptor expression may be a key mediator of COVID‐19 pathogenesis, featured as hyperinflammation and thrombosis. So far, there remains no clear antiviral treatments or vaccine against COVID‐19.
2. NSAIDs AND COVID‐19: FRIENDS OR FOES?
Although infection with SARS‐CoV‐2 can be asymptotic, the most common clinical symptoms for COVID‐19 usually start from fever, dry cough, fatigue, muscle pain, dyspnoea, anosmia, and ageusia days after catching the virus. Disease in some patients can then progress to severe/critical conditions and which regularly results in death. The risk factors for severe/critical disease include age, male sex, and co‐morbidities, but young COVID‐19 patients without underlying medical conditions can also develop severe disease (Huang et al., 2020; Oxley et al., 2020; Williamson et al., 2020). Compared to surviving patients, non‐surviving COVID‐19 patients had gradually and remarkably elevated inflammatory mediators in their blood, such as C‐reactive protein, D‐dimer, cytokines, for example, IL‐6, IL‐8, and TNF‐α, and neutrophil‐to‐lymphocyte ratio, progressing to ARDS, sepsis, and multi‐organ failure (D. Zhang, Guo, et al., 2020; F. Zhou, Yu, et al., 2020). This observation was confirmed by a systemic proteomic analysis of COVID‐19 patient sera (Shen et al., 2020), indicating a key role for hyperinflammation in COVID‐19 progression. Targeting inflammation is thus one of key strategies to manage COVID‐19 disease severity.
Non‐steroidal anti‐inflammatory drugs (NSAIDs), such as aspirin, ibuprofen, celecoxib, and indomethacin, are a group of medicines that are used very widely from managing acute (e.g., fever and pain) and chronic (e.g., rheumatoid arthritis) inflammatory conditions to preventing and/or treating cancers. NSAIDs are commonly used by COVID‐19 patients to reduce fever and relieve muscle pain, but whether NSAIDs are beneficial or harmful to COVID‐19 patients has become a hot topic. The use of NSAIDs thus far during the COVID‐19 pandemic remains controversial, and a cautionary approach is advised (Day, 2020; FitzGerald, 2020; Little, 2020). Indeed, NSAIDs are more likely to be regularly used by elderly people (>60 years) with existing health conditions such as high body mass index, increased waist circumference, or heart disease (Davis et al., 2017), which collectively represent risk factors for COVID‐19. Although the World Health Organization and the United Kingdom National Health Service have declared that there is no evidence of an increased risk of developing COVID‐19 or making the disease more severe by using NSAIDs, suggestions on avoiding NSAIDs for COVID‐19 patients were previously made based on numerous clinical trials and observations on non‐COVID‐19 pulmonary infectious diseases. For example, apart from the commonly adverse effects of NSAIDs such as gastrointestinal, renal, and cardiovascular complications (Bhala et al., 2013; Little et al., 2013), NSAIDs were found to cause more prolonged illness or complications when taken during respiratory tract infections (Le Bourgeois et al., 2016; Voiriot et al., 2019). It was reported that use of NSAIDs for fever or non‐rheumatologic pain during the early stages of infection increased the risk of severe bacterial superinfection (Micallef, Soeiro, & Jonville‐Béra, 2020). NSAIDs may increase hypercoagulation and the incidence of thrombosis due to decreased thrombomodulin (Rabausch et al., 2005; Schmidt et al., 2011), a particular concern given that COVID‐19 patients often have coagulation abnormalities and increased vascular clotting (Levi, Thachil, Iba, & Levy, 2020). It is plausible that NSAIDs may possibly inhibit protective host immune reactions against coronavirus replication (Wu et al., 2020) and enhance the proinflammatory cytokine storm observed in lungs of COVID‐19 patients, for example, through activation of inflammatory macrophages (Wu & Meydani, 2008). Moreover, as SARS‐CoV‐2 can infect human gut enterocytes (Lamers et al., 2020), it is important to know if NSAIDs synergise with SARS‐CoV‐2 infection to potentiate severe intestinal damage.
However, do NSAIDs have any potential beneficial effects on the risk of infection with SARS‐CoV‐2 and/or COVID‐19 severity? In a retrospective cohort study, Rentsch et al. (2020) found that exposure to NSAIDs (−365 to −14 days prior to baseline) was modestly associated with increased likelihood of COVID‐19 infection (multivariable odds ratio [OR] 1.27, 95% confidence interval [CI] 1.02–1.58), was not associated with hospitalisation (P = 0.19), and had a negative trend of association with intensive care (P = 0.08). In this study, information of NSAIDs only obtained from pharmacy records was counted, but individuals assigned as non‐NSAID users were still likely to get NSAIDs over the counter. In another study, Freites et al. (2020) found that use of NSAIDs was associated with reduced risk of hospital admission of COVID‐19 patients who had chronic inflammatory rheumatic disease (OR 0.37, 95% CI 0.15–0.91, P = 0.03). Castro, Ross, McBride, and Perlis (2020) reported that prescription of ibuprofen and naproxen (both NSAIDs) was also associated with reduced hospitalisation among 2,271 individuals who tested positive for COVID‐19 (OR 0.649, 95% CI 0.415–0.992 and OR 0.388, 95% CI 0.144–0.930, respectively) after adjustments for age, sex, race, ethnicity, site, and combined co‐morbidity index. Among hospitalised COVID‐19‐positive individuals, 29 out of 34 patients premedicated with ibuprofen (adjusted OR 0.421, 95% CI 0.139–1.046) and 6 out of 7 patients premedicated with naproxen (adjusted OR 0.362, 95% CI 0.019–2.269) did not require mechanical ventilation during hospitalisation (Castro et al., 2020). Huh et al. performed a large case–control study assessing the relationships of the risk of COVID‐19 and the use of various drugs in 65,149 adult subjects (>18 years old, 7.9% were tested COVID‐19 positive) using a national wide claims database of South Korea. They found that prior exposure to aspirin significantly associated with reduced risk of developing COVID‐19 (adjust OR 0.29, 95% CI 0.14–0.58, P < 0.001), while prior use of COX‐2 inhibitors did not significantly reduce the risk (Huh et al., 2020). Interestingly, Hong et al. (2020) have recently performed a pilot trial to treat COVID‐19 patients with celecoxib (Celebrex ®; a selective inhibitor of COX‐2) at either 0.2 g twice or once a day. The remission rates of COVID‐19 disease for full/high (0.2 g twice a day) and half/medium (0.2 g once a day) doses of celecoxib and control groups were 100%, 82%, and 57%, respectively (Hong et al., 2020). Celecoxib treatment also improved pulmonary opacification and pneumonia faster than control group based on chest CT scan results. This study suggested that celecoxib may promote the recovery of ordinary and severe cases of COVID‐19 and prevent the progression of severe disease to a critical stage (Hong et al., 2020). Low doses of celecoxib (i.e., 0.2 g once a day) used in this study was associated with a small increase in the risk (relative risk 1.35, 95% CI 1.00–1.82) of non‐fatal myocardial infarction, but the high dose of celecoxib (i.e., 0.2 g twice a day) was not associated with increased risk of myocardial infarction (relative risk 1.05, 95% CI 0.33–3.35) (García Rodríguez, Tacconelli, & Patrignani, 2008). These findings may thus be indicative of the safety and efficiency for use of such doses of celecoxib in COVID‐19 patients who have known cardiovascular conditions.
These (pharma)epidemiological and clinical observations suggest that NSAIDs may be beneficial in controlling the development of severe COVID‐19. The types and doses of NSAIDs determine their capability of inhibiting enzymic activities of COX‐1 or COX‐2 or both, which affects the production profile of downstream eicosanoids, resulting in varied side effects in the cardiovascular, gastrointestinal, renal, and other systems (Bhala et al., 2013; García Rodríguez et al., 2008; Little et al., 2013). However, the information for exposure of NSAIDs (e.g., drug types, doses, and exposure time) in the above epidemiological studies was unclear as they were mainly defined based on electronic health records. Lack of such information impedes further assessment of clinical benefits or harm of NSAIDs. Therefore, large‐scale, double‐blinded, randomised, and well‐controlled clinical trials are warranted to thoroughly assess the effects of specific NSAIDs at appropriate doses in prevention and treatment of COVID‐19 while also attempting to avoid their known side effects.
Moreover, another NSAID, indomethacin, has shown potential antiviral activity against human SARS‐CoV‐1 and canine coronavirus (Amici et al., 2006). Indomethacin does not affect coronavirus binding or entry into host cells but instead acts by blocking viral RNA synthesis in vitro. Oral administration of indomethacin (1 mg·kg−1·day−1 [equivalent ~40 mg·kg−1 for a 70‐kg human, less than the low‐medium dose of 75 mg daily used in adult patients] for 4 days starting on day 4 post‐infection) markedly reduced (by ~2–3 orders) shedding of canine CoV RNA in the faeces of dogs infected with canine CoV, but this antiviral effect was reversed upon suspension of indomethacin treatment (T. Xu, Gao, Wu, Selinger, & Zhou, 2020). Indomethacin has also been suggested to exhibit potent antiviral activity against SARS‐CoV‐2‐infected Vero E6 cells in vitro and canine CoV‐infected dogs in vivo (T. Xu, Gao, et al., 2020). A very recent study based on a multi‐stage model‐based approach showed that treatment with the sustained‐release formulation of indomethacin at the dose of 75 mg twice a day (high dose used clinically according to García Rodríguez et al., 2008) is expected to achieve a complete response in 3 days for the treatment in patients infected by SARS‐CoV‐2, suggesting that indomethacin could be considered as a promising candidate for the treatment of COVID‐19 (Gomeni, Xu, Gao, & Bressolle‐Gomeni, 2020; Koch et al., 2020). The antiviral capacity of indomethacin is conferred by activation of protein kinase R, independently of interferons and double‐stranded RNA (Amici et al., 2015) but may be via interactions with aldoketo‐reductases, aldose reductases, PPAR‐γ, and the cannabinoid CB2 receptor (T. Xu, Gao, et al., 2020). Conversely, it is important to report that indomethacin and other NSAIDs (including coxibs) are associated with nephrotoxicity (Delaney & Segel, 1985; McCarthy, Torres, Romero, Wochos, & Velosa, 1982; Whelton, 1999). As a result, such NSAIDs are not recommended for use in patients with clinically complicated SARS‐CoV‐2 infections with deficiencies in renal function, where renal blood flow is maintained by the contributory effects of vasodilator PGs (Grosser, Fries, & FitzGerald, 2006) or with gastrointestinal risk factors (Capuano et al., 2020). Therefore, such clinically complex SARS‐CoV‐2 infections constitute a contraindication to the use of NSAIDs as their use may predispose to these well‐known side effects of NSAIDs (Patrignani & Patrono, 2015). Importantly, indomethacin is also known to have coronary vasoconstrictor effects via blockade of vasodilatory PG synthesis or to a direct drug effect, suggesting that in patients with severe coronary‐artery disease, indomethacin should be used with caution (Friedman et al., 1981). Using an original virtual screening protocol, celecoxib at 50 μM (much higher than its maximum serum concentration of 1.8 μM when 200 mg·day−1 is used) was predicted to suppress the activity of the main chymotrypsin‐like protease (a key target for antiviral drugs) of SARS‐CoV‐2 by ~12% (Gimeno et al., 2020). In addition, naproxen has also been shown to possess antiviral activity against influenza A and B viruses by interfering with the RNA replication process (Zheng et al., 2019). Moreover, ibuprofen was reported to enhance ACE2 expression from diabetic rat cardiac tissues (Qiao et al., 2015), while celecoxib was shown to repress expression of TMPRSS2 in human prostate cancer cells (Kashiwagi et al., 2014). Given the protective action of ACE2 in ALI (Imai et al., 2008; Imai et al., 2005; Kuba et al., 2005; Kuba et al., 2006) and the negative correlation between ACE2 expression and SARS‐CoV‐2 severe outcomes (J. Chen, Jiang, et al., 2020), NSAIDs may help reduce COVID‐19 disease severity due to SARS‐CoV‐2 infection if they can up‐regulate and down‐regulate ACE2 and TMPRSS2 in the lung, respectively.
3. PGs IN THE HUMAN LUNG
NSAIDs serve to reduce inflammation by targeting cyclooxygenases (COXs, i.e., COX‐1 and COX‐2) and inhibiting biosynthesis of prostaglandins (PGs), a group of important lipid mediators. PGs are formed when arachidonic acid (AA) is released from cell membrane phospholipids by the actions of cytosolic PLA2 (cPLA2) and converted to PGH2 by COXs. COX‐1 is constitutively expressed in most cells, whereas COX‐2 is induced upon the initiation of inflammation (Dubois et al., 1998). PGH2 is unstable and converted into each PG by the corresponding specific synthases. Thus PGD2 is produced by the PGD synthases, LPGDS and HPGDS); PGE2 by the PGE synthases (mPGES‐1, mPGES‐2, and cPGES); PGF2α by the PGF synthases, including AKR1C3 and AKR1B1; PGI2 (prostacyclin) by the PGI synthase: and thromboxin A2 (TXA2) by TXA synthase (Figure 1). Single‐cell RNA sequencing analysis (Du et al., 2017) reveals that cPLAs, COXs, and PG synthases are expressed in normal human lung cells from a healthy young individual (Figure 2). For example, COX‐1 is expressed in immune cells such as dendritic cells (DCs) and mast cells while COX‐2 is broadly but moderately expressed by epithelial (e.g., AT1), stromal (e.g., matrix fibroblasts), and immune (e.g., monocytes/macrophages, DCs, and mast cells) cells. Endothelial, stromal, mast, DC, and NK cells highly express PGDSs, but only matrix fibroblasts and lymphatic endothelial cells moderately express mPGES‐1, the inducible enzyme that mediates PGE2 biosynthesis in vivo, in the normal lung.
FIGURE 1.
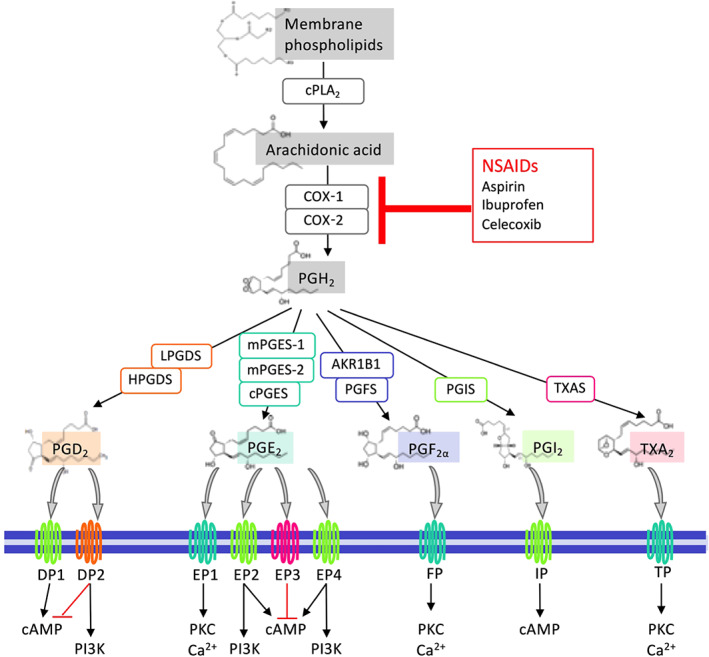
An overview of PG biosynthesis, receptors, and downstream signalling pathways. Arachidonic acid (AA) is released from membrane phospholipids via the actions of cytosolic PLA2 (cPLA2) following various stimuli and then metabolised to PGH2 by COXs (COX‐1 and COX‐2). PGH2 is unstable and subsequently converted into PGs, that is, PGD2, PGE2, PGF2α, PGI2, and TXA2 by the actions of their synthases PGDS (LPGDS and HPGDS), PGES (mPGES‐1, mPGES‐2, and cPGES), PGFS (AKR1B1 and PGFS/ABR1C3), PGIS, and TXAS, respectively. PGs bind to their receptors and activate different downstream signalling pathways. PGD2 receptors, DP1 and DP2, activate the cAMP and PI3K pathways, respectively, while DP2 also represses the cAMP pathway. PGE2 receptors EP2 and EP4 activate both cAMP and PI3K pathways, EP1 activates PKC and Ca2+ pathways, and EP3 deactivates the cAMP pathway. Both PGF2α receptor FP and TXA2 receptor TP activate PKC and Ca2+ pathways, whereas PGI2 receptor IP triggers activation of cAMP signalling. On the other hand, non‐steroidal anti‐inflammatory drugs (NSAIDs) inhibit AA biosynthesis of all PGs by targeting COX‐1 and/or COX‐2
FIGURE 2.
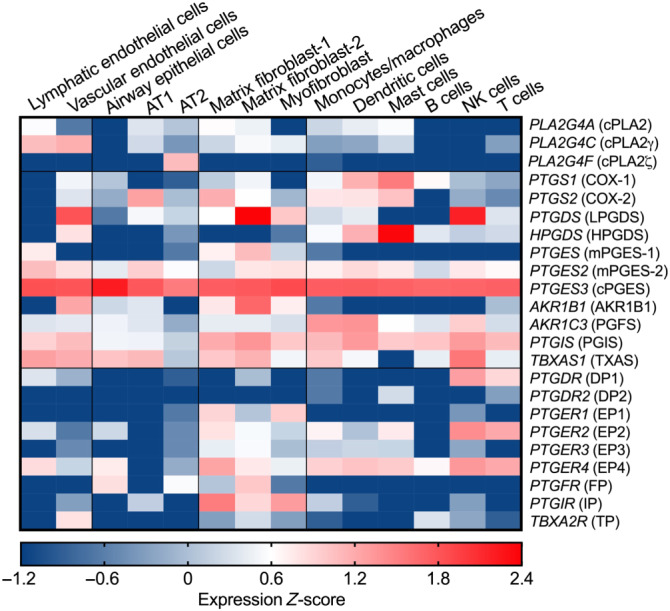
Gene expression profiles of PG synthases and their receptors in human lung cells. Gene expression data from single‐cell RNA sequencing analysis of healthy lung cells from a young individual were retrieved from LungGENS (Lung Gene Expression iN Single‐cell, Du et al., 2017) and transformed to Z‐score. AT1, type 1 alveolar epithelial cell (pneumocyte); AT2, type 2 alveolar epithelial cell (pneumocyte). Words in Italic represent human gene symbols, while words in brackets represent human protein names
PGs exert their autocrine and paracrine effects via activation of nine types and subtypes of rhodopsin‐like transmembrane spanning GPCRs. These are the PGE2 receptors (EP1, EP2, EP3 and EP4), PGD2 receptors (DP1 and DP2), PGF2α receptor (FP), PGI2 receptor (IP), and TXA2 receptor (TP). These PG receptors initiate multiple and distinct downstream signalling pathways (Figure 1) and thus function as double‐edged swords during diverse physiological and pathological processes where they can play conflicting roles (Narumiya & FitzGerald, 2001; Yao & Narumiya, 2019). In the healthy lung, NK and T cells express DP1 receptors while mast cells mildly express DP2 receptors. Among four PGE2 receptors, EP4 and EP2 receptors are expressed on stromal cells and nearly all immune cell types, but at very low levels on alveolar epithelial (AT1 or AT2) cells. IP receptors are highly expressed on stromal cells while TP receptors are expressed on vascular endothelial cells (Figure 2).
PGs are found in most mammalian cells and tissues and can promote both the initiation and resolution of inflammation, depending on local concentrations, disease setting, and timing of action (Yao & Narumiya, 2019). Fluctuations in expression of these synthases within cells recruited to sites of inflammation govern the production profiles of PGs. Although PGE2 is detectable in bronchoalveolar lavage (BAL) fluids (rather than bronchial wash fluids) taken from healthy individuals, its level (1.6 pM) is threefold to eightfold lower than levels of PGD2, PGF2α, and TXB2 (a stable TXA2 metabolite) (Gouveia‐Figueira et al., 2017). PG levels are usually elevated in response to inflammatory or noxious stimuli. For example, PGE2 compared to other PGs was found to be significantly up‐regulated in BAL fluids of individuals after exposure to biodiesel exhaust (Gouveia‐Figueira et al., 2017). Plasma levels of PGE2 were increased in patients from early stage (within the first week, 1,966 pg·ml−1 ≈ 5.6 nM) of SARS‐CoV‐1 infection and lasted to the late stage (2–3 weeks, 2,170 pg·ml−1 ≈ 6.2 nM) of infection compared to that in plasma from control individuals (1,300 pg·ml−1 ≈ 3.7 nM) (Lee et al., 2004). This is associated with induction of COX‐2 expression as the SARS‐CoV‐1 N protein binds directly to two regulatory elements (i.e., NF‐κB and C/EBP binding sites) of the COX‐2 promoter (Yan et al., 2006). By performing proteomic and metabolomic profiling of sera from COVID‐19 patients and healthy individuals, Shen et al. (2020) found a remarkable elevation of serum amyloid A1 (SAA1), SAA2, SAA4, and other inflammation markers (e.g., C‐reactive protein, SAP, and SERPINA3) in severe COVID‐19 patients compared to non‐severe COVID‐19 patients and healthy individuals. SAAs can enhance COX‐dependent AA metabolism and production of PGs, including PGE2, PGF2α, and TXA2, in many types of human cells, such as monocytes, macrophages, and fibroblasts, through NF‐κB activation (Li et al., 2017; Malle et al., 1997). Similarly, Yan et al. (2020) found up‐regulation of genes associated with the PGE2 synthesis pathway including PLAs, COX‐1, COX‐2, and PTGES3 during the disease progression of COVID‐19 patients, compared with that of healthy individuals in a longitudinal transcriptome analysis of peripheral blood mononuclear cells. In agreement with these findings, Hong et al. (2020) observed significantly higher (~10‐folds) levels of PGE2 in urine samples of COVID‐19 patients, compared with healthy individuals. PGE2 in COVID‐19 patient's urine remained at high levels for 5–12 days post‐hospital admission with routine treatment, but its level was reduced after taking oral celecoxib, a selective COX‐2 inhibitor. However, urinary PGE2 formed in the kidney does not represent systemic PG levels. To assess systemic production of PGs, detection of circulation levels of their metabolites, for example, PGE‐M, PGD‐M, and PGI‐M, in both blood and urine is needed. Moreover, PGs and their metabolites present in the lung, for example, in BAL fluid, will also be highly informative.
Traditionally, PGs have been viewed as inflammatory mediators connecting innate immunity to phases of acute inflammation with proinflammatory cytokines (TNF‐α and IL‐1β) and LPS known to induce expression of inducible COX‐2 and mPGES‐1 (Díaz‐Muñoz, Osma‐García, Cacheiro‐Llaguno, Fresno, & Iñiguez, 2010). However, COX‐2 and mPGES‐1 expression is also observed in chronically inflamed tissues including joints of rheumatoid arthritis patients, colons of patients with inflammatory bowel disease, and cancerous tumours and their micro‐environment (Ricciotti & FitzGerald, 2011; Wang & DuBois, 2018). Therefore, PGs appear to play integral roles during both acute inflammation and chronic inflammatory diseases. Gordon et al. systemically mapped the interaction landscape between SARS‐CoV2 proteins, including four structural (S, E, M, N) proteins and non‐structural proteins (Nsp), and human host cell proteins, and predicted numerous host proteins as potential drug targets. Remarkably, they found that non‐structural proteins Nsp7 and Nsp14 interacted with PTGES2 (encoding mPGES‐2) and PRKACA (encoding the catalytic subunit α of PKA), respectively (Gordon et al., 2020). mPGES‐2 mediates biosynthesis of PGE2, which mainly activates the cAMP–PKA pathway, and its receptors EP4 and EP2 are highly expressed in lung cells. Thus, targeting mPGES‐2 by NSAIDs (such as indomethacin, Yamada, Komoto, Watanabe, Ohmiya, & Takusagawa, 2005) can not only inhibit PGE2 production but also interrupt virus–host cell interaction. In addition, mPGES‐1 inhibition may be of clinical benefit during PGE2‐induced inflammatory episodes, including COVID‐19‐associated ARDS (Smeitink et al., 2020). Thus, targeting respective PG synthase might represent alternative and effective strategies in attempt to alleviate adverse side effects of NSAIDs that result from non‐specific inhibition of all PGs downstream of PGH2.
4. PGs IN COVID‐19: POSSIBLE IMMUNOPATHOLOGICAL MECHANISMS
What may be the underlying scientific rationale and biological mechanisms for either beneficial or harmful effects of NSAIDs on the risk of development of COVID‐19 or disease severity? What are the fundamental mechanisms underlying the risk factors (e.g., age, male sex, and underlying healthy conditions) for developing severe to critical disease and death in COVID‐19 patients (Docherty et al., 2020; Williamson et al., 2020)? Are PGs involved in the risks of development of severe COVID‐19 disease, and if yes, how do PGs fundamentally function at various stages of SARS‐CoV‐2 infection, virus–host interactions, and during pathogenesis of COVID‐19? Answers for such questions remain vague.
4.1. Potential roles of AA in COVID‐19
AA is known to have potent antimicrobial capacity including leakage and lysis of microbial cell membranes, viral envelope disruption, amino acid transportation, inhibition of respiration, and uncoupling of oxidative phosphorylation (Das, 2018). It is reasonable to suggest that a variety of immune cells, including neutrophils, alveolar macrophages, B and T lymphocytes, and NK cells, liberate AA and other unsaturated fatty acids to the immediate extracellular space when challenged by CoVs including SARS‐CoV‐1, MERS‐CoV, and possibly SARS‐CoV‐2 (Das, 2020). Indeed, Yan et al. (2019) found that HCoV‐229E infection markedly increased the levels of cPLA2‐dependent glycerophospholipids and fatty acids including Linoleic acid and AA and that supplementation with exogenous linolenic acid or AA inhibited virus replication of HCoV‐229E and MERS‐CoV in Huh‐7 cells. Accordingly, Shen et al. (2020) used metabolomic assays to show that serum levels of AA (20:4n6) were significantly reduced in COVID‐19 patients which was negatively associated with disease severity, suggesting that decreased levels of AA may lead to lack of inhibition of SARS‐CoV‐2 replication in COVID‐19 patients. In light of this, it is suggested that oral or intravenous administration of AA and other unsaturated fatty acids may enhance recovery in COVID‐19 patients (Das, 2020). On the other hand, decrease of AA levels could also be explained as a result of facilitation of AA metabolism, that is, conversion into PGs. Indeed, up‐regulated gene expression of COX‐1, COX‐2, and PTGES3 and increased PGE2 levels are found in COVID‐19 patients (Hong et al., 2020; Yan et al., 2020). Furthermore, cPLA2α serves to release of AA from cell membrane lipids and subsequent production of PGs. In the human lung, cPLA2 genes are highly expressed in endothelial and epithelial cells (Figure 2) where CoVs (SARS‐CoV‐1 and SARS‐CoV‐2) first attach. Expression of several PLA2 genes (e.g., PLA2G4A, PLA2G4C, PLA2G7, and PLA2G15) is up‐regulated in COVID‐19 patients compared to healthy controls, and their expression reduces to normal levels when patients recover from COVID‐19 (Yan et al., 2020). Inhibition of cPLA2α activity using pyrrolidine‐2 significantly reduced viral replication and formation of double membrane vesicles in HCoV‐229E‐infected Huh‐7 cells and MERS‐CoV‐infected Huh‐7/Vero cells (Müller et al., 2018), implying that anti‐CoV treatments harnessing cPLA2α inhibition may be of potential therapeutic benefit. It remains unknown whether cPLA2α and its products (i.e., fatty acids) regulate CoV replication directly or indirectly through their further downstream metabolites of fatty acids, for example, PGs. Below, we will focus on discussion of possible functions of PGD2 and PGE2 even though other PGs may also influence SARS‐CoV‐2 infection and COVID‐19. For example, PGI2 and TXA2 respectively can reduce and promote thrombosis, a typical complication that occurs in nearly half of critically ill COVID‐19 patients and which contributes to mortality (Klok et al., 2020; Wise, 2020). Moreover, the vasodilatory and endothelial cell effects of PGs are well known. However, here, we will focus on discussing the roles of PGs in COVID‐19 immunopathology.
4.2. Potential roles of PGs on thrombosis in COVID‐19
The exact mechanisms behind the development of systemic coagulopathy and acquired thrombophilia defined in the majority of COVID‐19 cases which can lead to venous, arterial, and microvascular thrombosis remain unclear (Becker, 2020). Indeed, clinical characteristics of COVID‐19 include elevated D‐dimer levels, prolonged thrombin time, and thrombocytopenia (platelet count <150,000·μl−1), therefore suggesting an increased possibility of disseminated intravascular coagulation or pre‐disseminated intravascular coagulation (Guan et al., 2020). Moreover, pooled analysis suggests that significant increases in D‐dimer levels as a predictor of adverse outcomes were regularly observed in COVID‐19 patient blood, implying the presence of underlying coagulopathy (Lippi & Favaloro, 2020). Although D‐dimer levels can be altered by numerous inflammatory processes, in cases of COVID‐19, it is almost certainly due to intravascular thrombosis (Cui, Chen, Li, Liu, & Wang, 2020; Leonard‐Lorant et al., 2020). A retrospective cohort study found that on admission, increased D‐dimer levels (>1,000 ng·ml−1) were associated with increased risk of death in hospitalised COVID‐19 patients (F. Zhou, Yu, et al., 2020). In COVID‐19 patients, increased D‐dimer levels continue to be a persistent marker of poor outcome (Bikdeli et al., 2020). Klok et al. (2020) reported a high incidence of 31% thrombotic complications in ICU patients with COVID‐19, of which venous thromboembolic events were most common (27%). Furthermore, in 183 consecutive patients with COVID‐19 pneumonia, abnormal coagulation parameters and poor prognosis were recorded, and patients who died (compared to survivors) exhibited increased D‐dimer levels, fibrinogen degradation products, and longer PT and APTT values (Tang, Li, Wang, & Sun, 2020). Pathological characteristics of COVID‐19 infection include platelet‐fibrin thrombosis and intravascular megakaryocytes in all major organs, including the heart and lungs (Fox et al., 2020). Megakaryocytes are responsible for the production of platelets; therefore, the employment of anti‐platelet agents may be of clinical benefit during COVID‐19 pathogenesis. Aspirin is a broadly studied anti‐platelet drug which exerts its cardioprotective effects via irreversible inhibition of platelet COX‐1, thus blocking TXA2 production in activated platelets, and so decreases prothrombotic events. However, aspirin does not confer platelet‐specific effects, and in other cell types (via inhibition of COX‐1 and in some cases COX‐2), it can decrease prostanoid production, for example, PGI2, which serves to inhibit platelet aggregation (conversely to TXA2). Interestingly, as determined by a pharmacodynamic interaction study in healthy volunteers, other NSAIDs including ibuprofen, naproxen, indomethacin, and tiaprofenic acid all block the anti‐platelet effect of aspirin, whereas celecoxib and sulindac did not exhibit any significant anti‐platelet effects (Gladding et al., 2008). Although aspirin can inhibit viral replication and confer anti‐inflammatory and anti‐coagulant effects, at present, it has not been thoroughly investigated in the treatment of thrombosis during COVID‐19. However, COVID‐19 clinical trials involving aspirin administration are ongoing. That said, PGs (whose production is blocked by NSAIDs) can also confer anti‐platelet effects and therefore also merit clinical attention in the context of COVID‐19. For example, it has been long understood that within the PG family, PGE1 is the most potent inhibitor of ADP‐induced platelet aggregation whereas PGE2 possesses roughly a fifth of its activity (Irion & Blombäck, 1969). PGE2–EP4 receptor signalling does effectively inhibit platelet aggregation at high concentrations (>1 × 10−6 M) (Macintyre & Gordon, 1975), and akin to PGI2, PGD2 can also inhibit platelet aggregation (Smith, Silver, Ingerman, & Kocsis, 1974), but PGE2–EP3 receptor signalling augments platelet aggregation (Friedman, Ogletree, Haddad, & Boutaud, 2015).
4.3. Potential roles of PGD2 in COVID‐19
Ageing is associated with up‐regulated gene expression of PG synthases (e.g., COX‐2 and mPGES‐1) and elevated levels of PGs (e.g., PGE2, PGD2, PGF2α, and TXB2) in humans (Li et al., 2015). Similarly, Vijay et al. (2015) found that expression of PLA2 group IID (PLA2G2D) was increased in aged mouse lungs compared to younger mice, leading to augmented production of PGD2, PGE2, PGF2α, and TXB2 in lungs in response to SARS‐CoV‐1 infection. Differential gene expression showed that the major source of PLA2G2D was lung‐resident CD11c+ cells, for example, alveolar macrophages and DCs (Vijay et al., 2015). Strikingly, aged mice with deficiency in PLA2G2D were protected from SARS‐CoV‐1 infection, exhibited enhanced virus‐specific cytotoxic CD8 T cell responses, and increased migration of respiratory DCs to draining lymph nodes (Vijay et al., 2015). PGD2 may contribute to SARS‐CoV‐1 infection, as treatment with exogenous PGD2 in young mice decreased respiratory DC migration, while treatment with a DP1 receptor antagonist enhanced respiratory DC migration, CD8 T cell responses, and the kinetics of virus clearance in lungs of aged, SARS‐CoV‐1‐infected mice (Zhao, Zhao, Legge, & Perlman, 2011). Enhanced T cell responses are of integral importance because reduced T cell responses are associated with higher mortality rates in SARS‐CoV‐1‐infected mice (Channappanavar, Zhao, & Perlman, 2014; Zhao, Zhao, Van Rooijen, & Perlman, 2009) and lymphopenia is also associated with disease progression of COVID‐19 (Tan et al., 2020). Vijay et al. (2017) further showed that PGD2–DP1 receptor signalling works together with type I IFN signalling to induce expression of the pyrin domain only‐containing protein 3 (PYDC3) that diminishes neurotropic CoV‐induced inflammasome activation and proinflammatory cytokine (such as IL‐1β) expression in mouse brain, thus preventing chronic inflammation and tissue damage. Interestingly, DP1 receptor signalling in human macrophages also up‐regulates POP3, a putative functional analogue of mouse PYDC3, suggesting that PGD2 similarly modulates inflammasomes in human cells (Vijay et al., 2017). These studies thus suggest that targeting the PGD2– DP1 receptor pathway may be helpful to control SARS‐CoV infection in older humans. Furthermore, SARS‐CoV‐2 may cause mast cell activation via the toll‐like receptors (TLRs) or inducing crosslink of IgE–FcεRI and subsequent production of PGD2, where use of mast cell stabilisers, including β‐adrenoceptor agonists, is highlighted as potential therapeutic targets during SARS‐CoV‐2 infection due to their efficient inhibition of PGD2 release (Kilinç & Kilinç, 2020). Infection with respiratory syncytial virus (RSV) up‐regulates HPGDS expression and increases PGD2 secretion by cultured human primary airway epithelial cells (Werder et al., 2018). Blocking the DP2 receptor decreased viral load, immunopathology, and morbidity in a neonatal mouse model of severe viral bronchiolitis (Werder et al., 2018). Interestingly, the beneficial effects of DP2 receptor antagonism are inhibited by concurrent blocking of the DP1 receptor, and DP1 receptor agonists up‐regulate type III IFN (i.e., IFN‐γ) production and IFN‐stimulated gene (ISG) expression, accelerating viral clearance (Werder et al., 2018). This finding suggests reciprocal effects of DP1 and DP2 receptors in RVS infection, although it is contrary to the findings of the pathogenic role of DP1 receptors during SARS‐CoV‐1 infection (Zhao et al., 2011). Therefore, investigations should attempt to analyse the actions of PGD2, DP1 receptors and DP2 receptors on SARS‐CoV‐2 infection and during development of COVID‐19.
4.4. Potential roles of PGE2 in COVID‐19
PGE2 is considered an anti‐inflammatory agent in various lung conditions including ALI, asthma, fibrosis, and bacterial infections (Birrell et al., 2015; Felton et al., 2018; Lundequist et al., 2010; Vancheri, Mastruzzo, Sortino, & Crimi, 2004). But it can also exert inflammatory effects in certain condition such as COPD, lung cancer, and certain viral infections (Bonanno et al., 2016; Dehle et al., 2013; Nakanishi & Rosenberg, 2013). Infection with a wide range of viruses, for example, herpes simplex virus, rotavirus, and influenza A virus (IAV), induces expression of COX‐2 and mPGES‐1, resulting in overproduction of PGE2, which in turn plays a role in viral infection directly by modulating viral binding, replication, and gene expression and/or indirectly by regulating the host immune responses (see Sander, O'Neill, & Pohl, 2017). Indeed, elevated levels of PGE2 were observed in patients infected with SARS‐CoV‐1 or SARS‐CoV‐2 (Hong et al., 2020; Lee et al., 2004). Furthermore, Smeitink et al. (2020) speculate that in males, increased PGE2 may correlate with enhanced COVID‐19 severity by augmenting thrombotic responses. This may be in part due to effects of PGE2 in promoting intravascular thrombosis via EP3 receptors in platelets (Gross, Tilly, Hentsch, Vonesch, & Fabre, 2007). Elevated levels of PGE2 may further enhance SARS‐CoV‐2 cell entry. Indeed, elevated PGE2 does enhance clathrin‐mediated endocytosis of bovine ephemeral fever virus (Cheng, Huang, Chi, Chiu, & Liu, 2015). Here, we will discuss potential actions of PGE2 in COVID‐19 based on the current state of the knowledge of this lipid in modulation of immune responses during infections or under inflammatory conditions (Figure 3).
FIGURE 3.
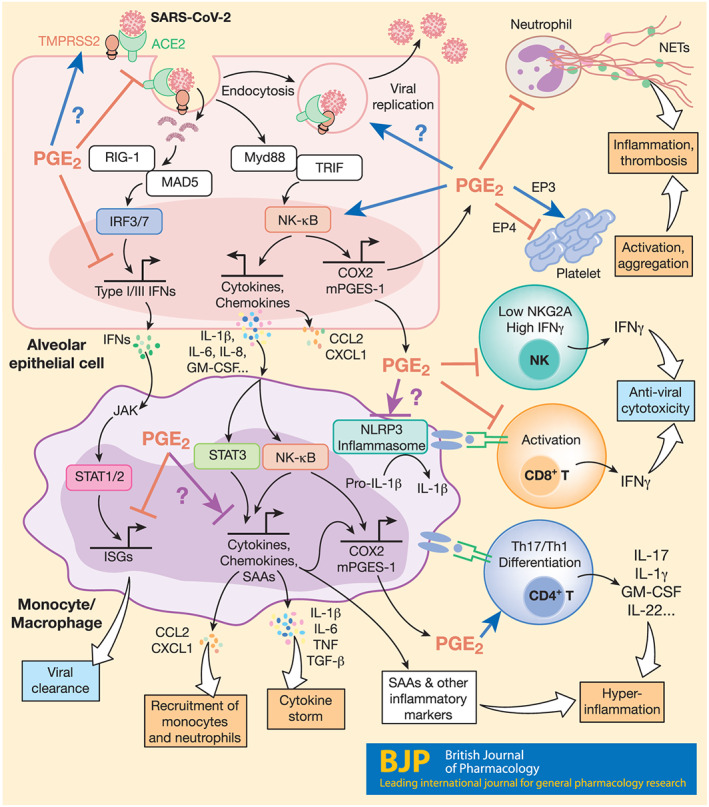
Possible mechanisms for PGE2 modulation of immune cell functions in COVID‐19. PGE2 is likely to modulate immune responses in various cell types during SARS‐CoV‐2 infection, influencing COVID‐19 pathogenesis. In epithelial cells, attachment of SARS‐CoV‐2 with ACE2 and TMPRSS2 leads to endocytosis, viral replication, and cell damage, activating RLR (RIG‐1 and MAD5)‐dependent production of type I and III IFNs and the TLR‐dependent NF‐κB pathway. The NF‐κB pathway induces expression of proinflammatory cytokines (e.g., IL‐1b, IL‐6, IL‐8, and GM‐CSF), chemokines (e.g., CCL2 and CXCL1), and other inflammatory mediators such as COX‐2 and mPGES‐1, resulting in PGE2 secretion. Here, while it suppresses production of type I (and possibly type III) IFNs, PGE2 further amplifies NF‐κB signalling and production of cytokines and chemokines in a positive feedback loop. PGE2 may also directly modulate ACE2 and TMPRSS2 gene expression, endocytosis, and viral replication. In monocytes/macrophages, activation of NF‐κB and STAT3 mediates production of large amounts of inflammatory cytokines which contributes to development of cytokine secretion syndrome (the “cytokine storm”), chemokines that recruit monocytes and neutrophils, inflammatory biomarkers (e.g., SAAs, CPR, and D‐dimer), and PGE2. Here, PGE2 again represses IFN‐induced expression of ISGs, contributing to delay of viral clearance. Importantly, PGE2 context‐dependently affects (either positively or negatively) not only NLRP3 inflammasome activation and related IL‐1β maturation but also NF‐κB‐dependent monocyte/macrophage cytokine production. PGE2 differentially regulates platelet aggregation via different receptors and probably inhibits NETosis associated with inflammation and thrombosis. PGE2 also down‐regulates IFN‐γ production and cytotoxicity of NK and CD8 T cells that kill cells infected with SARS‐CoV‐2 but promotes differentiation of proinflammatory Th17 and Th1 cells, the chief cellular sources of the cytokine storm at late stages of COVID‐19
4.4.1. PGE2 on IFN signalling
Infection with SARS‐CoV‐2 ligates various pathogen recognition receptors, for example, TLRs and/or RIG‐I‐like receptors, and activates transcription factors such as IFN regulatory factor 3 (IRF3) and NF‐κB that are responsible for expression of type I and III IFNs and proinflammatory mediators, including TNF‐α, IL‐6, and PGE2 respectively. Secreted IFNs then activate the JAK‐STAT1/2 pathway to trigger production of ISGs that directly recognise and execute antiviral functions (Park & Iwasaki, 2020). PGE2 has been reported to inhibit production of type I IFNs. For example, after infection with IAV, mPGES‐1 deficient macrophages exhibit an early increase in phospho‐IRF3, compared to wild‐type (WT) macrophages, augmented type I IFN, and decreased viral load, which was drastically inhibited by addition of exogenous PGE2 (Coulombe et al., 2014). Fabricius et al. (2010) reported that PGE2 inhibited secretion of IFN‐α by TLR‐activated human plasmacytoid DCs through its EP2 and EP4 receptor‐mediated suppression of IRF7. Patients with mild to moderate COVID‐19 had high type I IFN responses, while severe COVID‐19 patients had impaired type I IFN activity and down‐regulated ISGs (Hadjadj et al., 2020). It remains to be clarified whether severe COVID‐19 patients have higher levels of PGE2, and if so, whether this represents a mechanism evolved by SARS‐CoV‐2 for suppression of type I IFN production and function. Moreover, as DP1 receptors promote type III IFN production and signalling and contributes to RSV viral clearance (Werder et al., 2018) and PGE2 receptors (EP2 and EP4) both activate the cAMP–PKA pathway (as do DP1 receptors), it would be of interest to investigate whether PGE2 has different effects on production and functions of type I and III IFNs during SARS‐CoV‐2 infection.
4.4.2. PGE2 on NF‐κB signalling
Viral replication triggers hyperinflammation and cytokine storm syndrome, a leading cause of mortality in severe COVID‐19 patients (Mehta et al., 2020). Proinflammatory cytokines such as IL‐1, IL‐6, IL‐8, and TNF‐α and chemokines including CCL2, CCL3 and CXCL10 are produced from epithelial cells, monocytes, and macrophages, shortly after clinical symptoms appear (Mehta et al., 2020). Many clinical trials have been set up to treat COVID‐19 by targeting pathways relating to proinflammatory cytokine production, for example, IL‐1 and IL‐6 (Bonam, Kaveri, Sakuntabhai, Gilardin, & Bayry, 2020; Merad & Martin, 2020). NF‐κB is the key transcription factor responsible for induction of proinflammatory cytokines. Activation of NF‐κB can stimulate gene expression of inducible COX‐2 and mPGES‐1 in many cell types, leading to production of COX‐2‐dependent PGE2. This PGE2 acts autocrinally and/or paracrinally on NF‐κB stimulation for expanding of proinflammatory cytokines and chemokines through the EP2 (maybe also EP4) receptors (Aoki et al., 2017; Yao & Narumiya, 2019). For example, PGE2 mediates NF‐κB‐dependent IL‐8 gene transcription and protein secretion in human cells such as pulmonary microvascular endothelial cells, nasal polyp‐derived fibroblasts, and HEK293 cells, most likely through the EP4 receptor (Aso et al., 2012; Cho, Han, Lee, & Lee, 2014; Neuschäfer‐Rube, Pathe‐Neuschäfer‐Rube, Hippenstiel, Kracht, & Püschel, 2013). Thus, amplification of the PGE2–NK‐κB positive feedback system is likely to contribute to the cytokine storm and hyperinflammation observed in COVID‐19.
4.4.3. PGE2 on IL‐6 production
Elevated IL‐6 and IL‐6‐activated genes have been found in peripheral blood of COVID‐19 patients (Hadjadj et al., 2020; Huang et al., 2020). PGE2 has been reported to have contradictory effects on IL‐6 production which probably depends on the stimuli. For example, PGE2 acting on EP4 receptors inhibits LPS‐induced proinflammatory cytokines (e.g., IL‐6 and TNF‐α) and promotes systemic inflammation in multiple organs of mice (Duffin et al., 2016). Furthermore, PGE2– EP4 receptor signalling was also shown to suppress LPS‐induced ALI partially through IL‐6 and TNF‐α production (Birrell et al., 2015; Felton et al., 2018). PGE2 also inhibited LPS‐ and Streptococcus pneumoniae‐induced IL‐6 and TNF‐α production from human lung macrophages (Gill et al., 2016). Conversely, however, injection of mineral oils induced peritoneal macrophages to release IL‐6 and PGE2, which was reversed by inhibition of endogenous PGE2 signalling (Hinson, Williams, & Shacter, 1996). In humans, PGE2 promotes IL‐1β‐dependent production of IL‐6, M‐CSF, and VEGF from human fibroblasts via EP4 receptors (Inoue et al., 2002). PGE2 also enhances induction of IL‐6 and other proinflammatory cytokines (e.g., IL‐8) upon various stimuli in monocytes, macrophages, fibroblasts, and airway epithelial cells through both EP2 and EP4 receptors (B.‐C. Chen et al., 2006; Cho et al., 2014; Li, Qi, & Cowley, 2011; Raychaudhuri, Douglas, & Smith, 2010). In return, IL‐6 further up‐regulates COX‐2 gene expression and increases PGE2 production, working together for optimised production of other inflammatory factors, for example, MMP9 (Kothari et al., 2014). Moreover, PGE2 can promote IL‐6 production in a paracrine way. For example, PGE2 promotes Th17 cells to secrete IL‐17A which then stimulates fibroblast to produce proinflammatory cytokines, for example, IL‐6, IL‐8, and IL‐1β (Paulissen et al., 2013).
4.4.4. PGE2 on inflammasome activation
Inflammasomes play a critical role in the formation of cytokine storms commonly observed in SARS patients and possibly have similar functions in COVID‐19 patients. SARS‐CoV‐1 E protein activates not only NF‐κB but also the inflammasome NLRP3 (NOD‐, LRR‐, and pyrin domain‐containing protein 3), leading to secretion of IL‐1β and IL‐18 (Nieto‐Torres et al., 2015). SARS‐CoV‐1 open reading frame (ORF) 3a and ORF8b can also activate NLRP3 inflammasome (C.‐S. Shi, Nabar, Huang, & Kehrl, 2019; Siu et al., 2019). It was reported that SARS‐CoV‐2 S protein primes NLRP3 inflammasome activation and IL‐1β secretion in macrophages derived from COVID‐19 patients but not in macrophages from healthy SARS‐CoV‐2 naïve controls and that chemical inhibition of NLRP3 blocks spike protein‐induced IL‐1β secretion ex vivo (Theobald et al., 2020). PGE2 promotes pro‐IL‐1β gene expression and IL‐1β secretion via a positive feedback loop (Zasłona et al., 2017). In a mouse model of Pseudomonas aeruginosa bacterial infection, PGE2 mediates IL‐1β induction, limits autophagy‐mediated killing of P. aeruginosa in alveolar macrophages, and augments IL‐1β‐mediated ALI (Martínez‐Colón, Taylor, Wilke, Podsiad, & Moore, 2018). But there are contradictory findings on how PGE2 modulates NLRP3 inflammasome activation. PGE2 was reported to inhibit NLRP3 inflammasome activation in macrophages through EP4 receptors, and blockade of COX‐2 or EP4 receptors resulted in increased NLRP3 inflammasome activation (Mortimer, Moreau, MacDonald, & Chadee, 2016; Sokolowska et al., 2015). However, other authors report positive effects of PGE2 upon NLRP3 inflammasome activation and IL‐1β production in macrophages and monocytes under various stimuli (Nakata et al., 2020; Sheppe et al., 2018; Zasłona et al., 2017). Taken together, these findings indicate context‐dependent modulation of NLRP3 inflammasome activation by PGE2. Careful examinations are thus required to clarify the effects of PGE2 upon NLRP3 inflammasome activation, for example, primed by different SARS‐CoV‐2 proteins in different cell types.
4.4.5. PGE2 on monocyte/macrophage functions
Monocytes and macrophages are main sources of proinflammatory and anti‐inflammatory cytokines and generally function to eliminate pathogens. Expansion of IL‐6‐producing CD14+CD16+ monocytes was observed in peripheral blood from severe COVID‐19 patients (X. Zhang, Tan, et al., 2020; Y. Zhou, Fu, et al., 2020), but reduction of HLA‐DR on CD14+ monocytes was found in COVID‐19 patients with severe respiratory failure, which was associated with increased IL‐6 (Giamarellos‐Bourboulis et al., 2020), indicative of immunosuppression. This scenario is likely to involve the immuno‐suppressant PGE2 that can be secreted by both human monocytes and macrophages. While human macrophages use an IL‐1β‐independent pathway exclusively, monocytes produce PGE2 by both IL‐1β‐independent and IL‐1β‐dependent pathways; the latter involving TLR4/TRIF/IRF3 signalling (Endo, Blinova, Romantseva, Golding, & Zaitseva, 2014). Qiu et al. (2017) found an increase of CD14+CD16+ monocytes in patients with severe sepsis or septic shock, which was positively associated with disease severity. After in vitro culture of monocytes from sepsis patients, PGE2 diminished CD14+CD16+ monocytes after 24 h, reduced TNF‐α production, but enhanced anti‐inflammatory IL‐10 production (Qiu et al., 2017). High amounts of IL‐1β and PGE2 are mainly produced from the classic inflammatory CD14+CD16− human monocytes after Candida albicans infection (Smeekens et al., 2011). Increase in inflammatory monocytes was indicated to be associated with increased survival rate at least in Gram‐negative sepsis patients (Gainaru et al., 2018). Hence, reduction of inflammatory CD14+ monocytes by PGE2 may sustain immunosuppression and could be associated with poor clinical outcome. Given the important roles of monocytes and their abilities to differentiate into macrophages and DCs, it is imperative to examine the effects of PGs on functions of each subpopulations of monocytes under different stimulatory conditions of SAR‐CoV‐2 infection.
Single‐cell RNA sequencing analysis of cells in BAL fluid from COVID‐19 patients suggested that monocyte‐derived macrophages, but not alveolar macrophages, contribute to lung inflammation and damage in severe COVID‐19 patients (Liao et al., 2020). Bulk RNA sequencing analysis of BAL cells also suggested increased production of chemokines such as CCL2 and CXCL1 (Liao et al., 2020; Xiong et al., 2020; Z. Zhou, Ren, et al., 2020), which recruit CCR2‐expressing classical monocytes and neutrophils, respectively, to the lung from peripheral blood. Interestingly, PGE2–EP2 receptor signalling increases CCL2 and CXCL1 production from macrophages and other cells (Aoki et al., 2017; Yao & Narumiya, 2019). During human monocyte/macrophage differentiation, cAMP‐elevating reagents such as PGE2 can cause a large increase in the mRNA and protein levels of several proinflammatory CCL and CXCL chemokines, contributing to the pathogenesis of lung disease (Hertz et al., 2009). Furthermore, alternatively activated M2 macrophages differentiated from monocytes promote tissue repair by secreting reparative cytokines such as TGF‐β, amphiregulin (AREG) and VEGF (Wynn & Vannella, 2016). As PGE2 facilitates generation of M2 macrophages, it may thus also contribute to lung fibrosis in severe COVID‐19 patients.
4.4.6. PGE2 on NET release
The formation of neutrophil extracellular traps (NETs) is an evolutionarily ancient process which involves the release of de‐condensed nuclear chromatin studded with various antimicrobial proteins, such as core histones, neutrophil elastase and MPO, to the extracellular space where they serve to trap and kill invading microorganisms (Brinkmann et al., 2004; Fuchs et al., 2007; Robb, Dyrynda, Gray, Rossi, & Smith, 2014). However, aberrant NET formation is involved in a wide range of NET‐associated diseases. Hence, NETs are regarded as double‐edged swords in innate immunity (Kaplan & Radic, 2012). Given the important role played by NETs in the pathogenesis of various respiratory diseases and thrombosis, many researchers also suggest NETs as key players in the pathogenesis of COVID‐19, most probably via NET‐mediated release of excessive amounts of IL‐6 and IL‐1β during cytokine storms in the COVID‐19 milieu (Barnes et al., 2020; Mozzini & Girelli, 2020; Thierry, 2020; Thierry & Roch, 2020; Tomar, Anders, Desai, & Mulay, 2020). Indeed, there is a high degree of NET–IL‐1β interplay during both venous and arterial thrombosis and severe asthma (Lachowicz‐Scroggins et al., 2019; Liberale et al., 2019; Yadav et al., 2019), and it has been hypothesised that there may be therapeutic potential in targeting the IL‐1β/NET feedback loop (Yaqinuddin & Kashir, 2020). It is proposed that upon SARS‐CoV‐2 infection, activated endothelial cells recruit neutrophils where they release NETs, which in turn activates the contact pathway of coagulation, subsequently trapping and activating platelets to potentiate blood clotting (Merad & Martin, 2020). NETs contribute to immunothrombosis in COVID‐19 ARDS where pulmonary autopsies confirmed NET‐associated microthrombi with neutrophil–platelet infiltration (Middleton et al., 2020). Such NET‐induced immunothrombosis may help explain the prothrombotic clinical presentations observed in COVID‐19 patients (Middleton et al., 2020). Indeed, NETs have been identified as potential markers of disease severity in COVID‐19 (Zuo et al., 2020), and elevated NET formation in hospitalised COVID‐19 patients is associated with higher risk of thrombotic episodes (Zuo et al., 2020). Serum samples of hospitalised COVID‐19 patients contained greater cell‐free DNA and hallmark NET‐associated products, including MPO–DNA complexes and citrullinated histone H3, compared to healthy control serum samples (Zuo et al., 2020). Furthermore, serum of COVID‐19 patients requiring mechanical ventilation exhibited augmented cell‐free DNA and MPO–DNA complexes, compared to patients breathing room air (Zuo et al., 2020). An additional NET marker, calprotectin, was found to be present at prominently elevated levels in the blood of 172 COVID‐19 patients (H. Shi, Zuo, et al., 2020). PGs are reported to have inhibitory effect upon NET formation. PGE2 inhibited NET formation after stem cell transplant (Domingo‐Gonzalez et al., 2016) and via EP2‐ and EP4 receptor‐mediated activation of cAMP (Shishikura et al., 2016). After co‐culture of neutrophils with cAMP‐elevating reagents, PMA‐induced NET formation was significantly reduced (Shishikura et al., 2016). The adenylate cyclase toxin which vastly increases intracellular cAMP is also known to reduce NETs (Eby, Gray, & Hewlett, 2014). Interestingly, induction of intracellular cAMP production by PGE1 markedly constrains NETs induced by the pancreatic cancer cell line AsPC‐1 (Jung et al., 2019). Importantly, CGS21680, a selective agonist of the adenosine A2A receptor (which increases intracellular cAMP), successfully diminished NET formation mediated by antiphospholipid antibodies, which increased the incidence of thrombotic events (Ali et al., 2019). Furthermore, in mice treated with antiphospholipid antibodies, CGS21680 impaired thrombosis within the inferior vena cava (Ali et al., 2019). Here, the authors also demonstrate similar inhibition of NETs via dipyridamole, an antithrombotic medication which increases extracellular adenosine and impedes cAMP breakdown (Ali et al., 2019). Together with the known inhibitory effects of PGE2 on platelet function (Gross et al., 2007), such evidence suggests that there may be the potential for therapeutic gain in harnessing existing drugs that augment production of PGs and cAMP and thus reduce excess NET formation and the incidence of thrombotic events during SARS‐CoV‐2 infection.
4.4.7. PGE2 on T cell functions
T cells may play critical roles in SARS‐CoV‐2 infection and COVID‐19 pathogenesis. First, CD8+ cytotoxic T cells attack infected cells to prevent viral amplification. Second, CD4+ helper T cells help B cells produce antibodies and modulate innate immune responses. Third, hyperactivated and differentiated type 1 and 17 helper T cells (i.e., Th1 and Th17 cells, respectively) produce large amounts of proinflammatory cytokines (e.g., IL‐2, TNF‐α, IFN‐γ, IL‐6, and IL‐17) that contribute to the cytokine storm and immunopathology, but viral infection may also activate regulatory T (Treg) cells which limit immunopathology through mechanisms such as the production of anti‐inflammatory cytokines (e.g., IL‐10). After infection with SARS‐CoV‐2, a significant reduction of peripheral blood CD4+ and CD8+ T cells (a condition known as lymphopenia) results in moderate to severe COVID‐19 patients, which is correlated with disease severity and mortality (G. Chen, Wu, et al., 2020; Diao et al., 2020; Liu, Li, et al., 2020; Tan et al., 2020). In contrast to peripheral blood, mass lymphocyte infiltrations were observed in the lung as confirmed by post‐mortem examination of a COVID‐19 patient who suffered from ARDS (Tian et al., 2020). PGE2 has multifaceted effects on modulation of T cell responses (Yao & Narumiya, 2019). PGE2 suppresses T cell receptor‐dependent T cell activation and proliferation via EP2/EP4 receptor‐mediated cAMP–PKA pathway, but this suppressive effect is weakened by enhancing CD28 co‐stimulation through augmentation of PI3K signalling (Yao et al., 2013). Following SARS‐CoV‐2 infection, the pathway related to CD28 signalling in T helper cells was significantly down‐regulated, while the PKA pathway and PGE2 biosynthesis pathway were significantly up‐regulated (Z. Zhou, Ren, et al., 2020; Yan et al., 2020). Thus, PGE2–cAMP–PKA signalling is likely to inhibit antigen‐dependent activation of antiviral T cell responses in COVID‐19 patients. On this basis, use of NSAIDs by inhibiting endogenous PGE2 production may enhance antiviral T cell responses in COVID‐19 patients. Indeed, enhanced viral antigen‐specific CD8+ and CD4+ T cell responses in lungs were found in PTGES‐deficient mice where PGE2 production was largely reduced, compared to WT mice, post‐IAV infection, and this was associated with reduced viral load in PTGES‐deficient animals (Coulombe et al., 2014). Moreover, increased expression of the immune checkpoint proteins, PD‐1 (CD279) and TIM‐3 (CD366), on CD8+ T cells was detected in severe and critical COVID‐19 patients (Diao et al., 2020; Y. Zhou, Fu, et al., 2020), suggesting that T cell exhaustion may occur. Interestingly, PGE2 through EP2 and EP4 receptors synergies with PD‐1 signalling to suppress mice and human cytotoxic T cell survival and function during chronic viral infection or in the tumour micro‐environment. Combined blockade of COX–PGE2 signalling and PD‐1/PD‐L1 augments T cell responses and improves control of viral infection and tumour growth (J. H. Chen et al., 2015; Miao et al., 2017; Sajiki et al., 2019).
As for inflammatory T cells, PGE2 regulates Th1 cell differentiation dependently on the strengths of T cell receptor and CD28 co‐stimulation and timing of PGE2 encounter (Yao et al., 2013). Given the down‐regulation of CD28 signalling, immediate up‐regulation of gene‐related PGE2 synthases (i.e., PTGS1, PTGS2, and PTGES3) at the early stages post‐SARS‐CoV‐2 infection, and the kinetics of PGE2 secretion in COVID‐19 patients (Hong et al., 2020; Yan et al., 2020; Z. Zhou, Ren, et al., 2020), it is proposed that PGE2 may inhibit the development of inflammatory IFN‐γ‐producing Th1 cells, although further investigations are required. This possibility is further supported by the findings that PGE2 inhibits monocyte‐derived DCs and macrophages to produce IL‐12 (Kaliński, Hilkens, Snijders, Snijdewint, & Kapsenberg, 1997; van der Pouw Kraan, Boeije, Smeenk, Wijdenes, & Aarden, 1995), the key cytokine for generating IFN‐γ‐producing Th1 cells. Of note, IFN‐γ production from CD8+ T cells and NK cells was not significantly different in blood of non‐severe and severe COVID‐19 patients, but IFN‐γ+ CD4+ T cells were likely to be reduced in severe, compared to non‐severe, COVID‐19 patients (G. Chen, Wu, et al., 2020; Qin et al., 2020). Th17 cells highly express IL‐17A, IL‐17F, IL‐22, and GM‐CSF that contribute to COVID‐19 immunopathology. PGE2 stimulates IL‐17 and aryl hydrocarbon receptor signalling pathways in human and mouse T cells through EP2/EP4 receptor –cAMP–PKA signalling, necessary for generation of pathogenic Th17 cells (Boniface et al., 2009; Lee et al., 2019; Robb et al., 2018; Yao et al., 2009). Given that SARS‐CoV‐2 infection specifically up‐regulates IL‐17F signalling and aryl hydrocarbon receptor signalling (Z. Zhou, Ren, et al., 2020), PGE2 is assumed to promote inflammatory Th17 cell expansion and function in COVID‐19.
There are mixed findings regarding Treg cells in COVID‐19. Reduction of Treg cell frequencies was observed in severe COVID‐19 patients compared to non‐severe patients (G. Chen, Wu, et al., 2020; Qin et al., 2020), but Y. Shi, Tan, et al. (2020) reported an increase in Treg cell numbers in peripheral blood of mild COVID‐19 patients compared to the control group, and there was no difference in Treg cell numbers between mild/moderate and severe patients. Both stimulatory and inhibitory effects of PGE2 on human Treg cell generation and suppressive function were observed (Li et al., 2019; Schiavon et al., 2019). The definitive functions of PGE2 on Treg cells in peripheral blood and lungs of COVID‐19 patients remain to be determined.
4.4.8. PGE2 on NK cell functions
Like T cells, NK cells are also depleted in peripheral blood of severe, but not mild, COVID‐19 patients (Wen et al., 2020; Wilk et al., 2020; Zheng et al., 2020). NK cell activation may also be impaired in COVID‐19 patients due to down‐regulation of CD107a and cytokines such as IFN‐γ and TNF‐α but increased expression of NKG2A/CD94 that inhibits NK cell cytotoxicity (Zheng et al., 2020). These studies suggest that NK cell numbers and function are impaired in severe COVID‐19 patients. PGE2 was found to inhibit not only NK cell production of IFN‐γ but also myeloid cell production of IL‐12 that is required for IFN‐γ production through EP4 receptors (Van Elssen et al., 2011). The PGE2– EP4 receptor–cAMP signalling pathway also suppresses the cytolytic activity of NK and CD8+ T cells by increasing expression of NKG2A/CD94 (Holt, Ma, Kundu, & Fulton, 2011; Park et al., 2018; Zeddou et al., 2005). Moreover, PGE2 inhibits CXCR3 ligands such as CXCL9 and CXCL10 from antigen‐presenting cells, preventing NK cell migration (Gustafsson et al., 2011). It is thus likely that PGE2 both directly and indirectly inhibits NK cell migration to the lung, their activation and cytotoxic function, in the context of COVID‐19. However, this remains to be confirmed.
4.5. Potential roles of PGI2 in COVID‐19
Most human lung cells including stromal (fibroblasts), immune (monocytes, macrophages, and lymphocytes), and vascular endothelial cells express PGI2 synthase (PGIS) and the PGI2 receptor IP (Figure 2). As PGI2 also activates the cAMP–PKA signalling pathway, as does PGE2–EP2/EP4 receptor signalling, it is assumed that these two molecules may share similar effects on modulation of immune and inflammatory responses (see Dorris & Peebles, 2012) although PGI2 has been less extensively studied, compared with PGE2. Firstly, PGI2 is likely to suppress virus (e.g., RSV)‐induced type I IFN production in the lung, which contributes to protection against viral infection (Hashimoto et al., 2004; Toki et al., 2013). Overexpression of PGIS in bronchial epithelium decreased viral replication and limited weight loss while IP deficiency exacerbated RSV‐induced weight loss with delayed viral clearance and had greater IFN‐α and β protein expression post‐RSV challenge (Hashimoto et al., 2004; Toki et al., 2013). Secondly, like PGE2, PGI2–IP signalling also synergises with inflammatory cytokines (e.g., TNF‐α and IL‐1β)‐activated NF‐κB for production of IL‐6 in stromal cells (Honda, Segi‐Nishida, Miyachi, & Narumiya, 2006). However, opposite to the effects of PGE2, PGI2 also appeared to down‐regulate TNF‐α and CCL2 and thus to reduce recruitment of CCR2‐expressing monocytes and macrophages to inflammation sites (Kumei et al., 2018). Thirdly, PGI2 modulated adaptive T cell responses, for example, promoting inflammatory IFN‐γ‐producing Th1 and IL‐17‐producing Th17 cells (Nakajima et al., 2010; Truchetet, Allanore, Montanari, Chizzolini, & Brembilla, 2012; W. Zhou et al., 2012). Lastly, PGI2 stimulated Ca2+ efflux through IP‐activated cAMP, counteracting platelet activation (e.g., induced by TXA2), which is also akin to effects of the PGE2– EP4 receptor pathway. The ability of PGI2 to regulate immune and inflammatory responses thus has implications for COVID‐19.
4.6. Potential effects of clinical interventions of COVID‐19 on PG biosynthesis
There are many therapeutic strategies to combat COVID‐19 that have been attempted in a number of clinical trials. The main targets include (1) SARS‐CoV‐2 attachment and entry to human cells (e.g., the TMPRSS2 inhibitor camostat mesylate and viral fusion inhibitors such as hydroxychloroquine) and viral replication (e.g., remdesivir and lopinavir); (2) systemic immune responses, for example, biological agents, such as anti‐IL‐6, anti‐TNF, anti‐IL‐1β, anti‐IFN‐γ, or low MW inhibitors; and (3) hyperinflammation such as steroids,such as dexamethasone and NSAIDs. Trials using antibodies targeting cytokines or chemokines aim to block inflammatory immune cell migration and function and subsequently to reduce the cytokine storm‐induced systemic inflammation. As a result, down‐regulation of inducible COX‐2 and mPGES‐1 as well as PG biosynthesis is also expected, as discussed above. Apart from the small pilot trial administering celecoxib to COVID‐19 patients (Hong et al., 2020), another clinical trial has been registered as of July 9, 2020, proposing to use naproxen in critically ill COVID‐19 patients (https://clinicaltrials.gov/ct2/show/NCT04325633). As an inhibitor of both COX‐2 and IAV nucleoprotein, naproxen reduced mortality of patients with H3N2 influenza infection when administered in combination with clarithromycin and oseltamivir in a recent clinical trial (Hung et al., 2017). It is thus expected that naproxen may reduce the mortality associated with critical COVID‐19 patients via both COX‐independent antiviral transcription/replication and COX‐2‐dependent anti‐inflammatory effects (https://clinicaltrials.gov/ct2/show/NCT04325633). Although corticosteroids are not recommended for treatment of SARS (Stockman, Bellamy, & Garner, 2006), there are many clinical trials of steroids in COVID‐19 patients (https://clinicaltrials.gov). Strikingly, a very recent large‐scale clinical trial showed that low‐dose dexamethasone (6 mg once daily) reduced deaths by one third in ventilated patients with COVID‐19 and by one fifth in other COVID‐19 patients who received oxygen only, while no benefit was observed in COVID‐19 patients not requiring respiratory support (Horby et al., 2020). Given that corticosteroids including dexamethasone inhibit PG (especially PGE2) production from various human tissues such as gut and lung (Aksoy, Li, Borenstein, Yi, & Kelsen, 1999; Hawkey & Truelove, 1981; Ogushi, Ozaki, Kawano, & Yasuoka, 1987), results from this clinical trial are promising for COVID‐19 therapeutic strategies targeting PG pathways. Corticosteroids suppress PG production through inhibition of the enzymic activities of PLA2 and COX‐2, but not COX‐1 (M.‐Z. Zhang, Harris, & McKanna, 1999). Dexamethasone represses COX‐2 expression by both transcriptional and post‐transcriptional mechanisms; that is, dexamethasone reduces COX‐2 gene expression through thyroid hormone receptors interfering the binding of NF‐κB to the COX‐2 gene promoter and decreases COX‐2 mRNA stability via mechanisms involving preferential loss of polyadenylated mRNA, resulting in switching off mRNA translation and protein synthesis (Barnes, 2006; Newton, Seybold, Kuitert, Bergmann, & Barnes, 1998). Of note, despite inhibition of COX‐2 by both NSAIDs and steroids, they have different in vivo actions in managing inflammatory diseases. For example, while dexamethasone only had moderate effects on reducing mortality of severe/critical (but not non‐severe) COVID‐19 patients (Horby et al., 2020), celecoxib reduced disease severity and improved remission in both non‐severe and severe COVID‐19 patients (Hong et al., 2020), indicating different clinical outcomes from use of NSAIDs and other anti‐inflammatory agents. Nevertheless, further studies are essential to clarify the clinical safety and efficiency of these two families of anti‐inflammatory drugs and to understand the underlying immunomodulatory mechanisms.
5. CONCLUSIONS
Here, we have discussed the potential actions of PGs in SARS‐CoV‐2 infection and COVID‐19 pathogenesis based on the analysis of how PGs work in other inflammatory conditions and infections, such as SARS‐CoV‐1 and MERS‐CoV, including the potential positive and negative effects of NSAIDs during COVID‐19 (Figure 4). While concerns were raised for ibuprofen use in COVID‐19 patients, epidemiological studies have suggested that benefits for NSAID use are likely, by reducing the risk of development of severe disease in COVID‐19 patients. This was supported by a pilot experimental trial in which celecoxib reversed COVID‐19 disease development and progression to a severe state. Further epidemiological analyses and large‐scale clinical trials are needed to clarify the effects of NSAIDs (and their associated risks) during SARS‐CoV‐2 infection and COVID‐19 disease severity. Substantial evidence suggests that PGs are likely to be involved in many stages of SARS‐CoV‐2 infections and the development of COVID‐19, although their actual role(s) remain to be elucidated. For example, PGs and their precursors may affect SARS‐CoV‐2 attachment by modulating ACE2 and TMPRSS2 expression and virus endocytosis by regulating lipid vesicle fusion. Importantly, PGs are more likely to modulate host immune and inflammatory responses activated by virus pathogens. PGs, especially PGE2, can foster or restrain both innate and adaptive immune reactions. For example, PGE2 can repress type I IFN signalling, cytotoxic T cell responses, NET formation, inflammasome activation, and inflammatory cytokine production, whereas it can also contribute to inflammatory Th17 responses, NF‐κB activation, and related inflammatory cytokine production. These effects of PGs rely, to a great extent, on the context and micro‐environments such as strength (e.g., viral load) and timing of stimuli, organ locations, and responding cell types. Given the critical role of cytokine storms in COVID‐19 immunopathology and the context‐dependent regulation of cytokine production by PGs, it is imperative to understand the chief cellular sources of cytokines in the lung and peripheral blood, the key stimuli (e.g., SARS‐CoV proteins or peptides) and to elucidate the kinetics of cytokine secretion. Results from such studies may be insightful for considerations on when exactly NSAIDs could be administered to COVID‐19 patients to tackle hyperinflammation while minimising their adverse side effects. Furthermore, as NSAIDs non‐selectively block all PG pathways by targeting COXs (that may be related to unfavourable side effects), targeting respective PG synthesis or PG receptors may help to avoid some of the adverse side effects of NSAIDs. By and large, current evidence is insufficient to support if PGs or, in other words, NSAIDs have beneficial or deleterious actions on SARS‐CoV‐2 infection, development, and disease progression, although PGE2 (maybe also PGD2) is likely to be involved in inhibiting host antiviral responses and development of hyperinflammation in patients with COVID‐19. In light of the evidence detailed in this review, it is imperative to obtain comprehensive understanding of which immune response(s) could predominate at each stage of COVID‐19, and how PGs modulate different cell type‐dependent anti‐inflammatory and proinflammatory responses during the course of COVID‐19. Such insights will improve clinical understanding, especially in attempting to decide when and what specific NSAID could be administered at what dose ranges during disease progression in COVID‐19 patients, taking into careful consideration any co‐morbidities, as well as the known side effects of NSAIDs.
FIGURE 4.
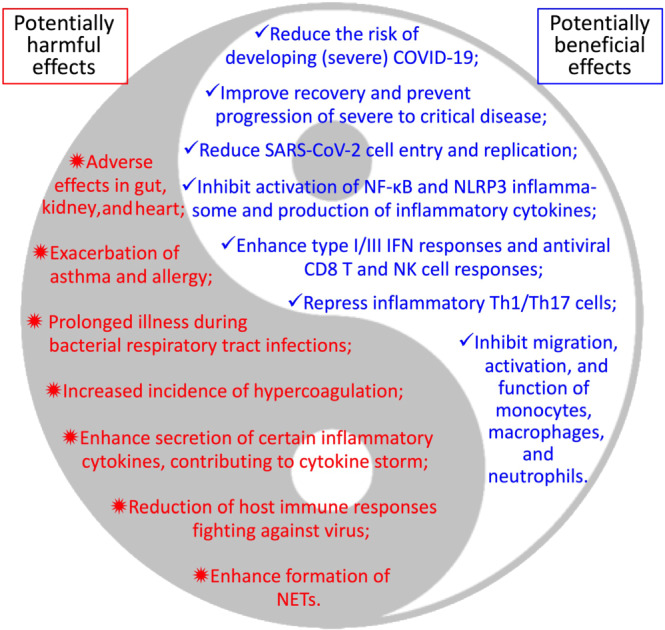
Yin and Yang of NSAIDs in COVID‐19: potential effects. A diagram showing potentially positive and negative effects of NSAID use in COVID‐19 patients
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, et al., 2019; Alexander, Fabbro, et al., 2019a, b; Alexander, Kelly, et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
A.G.R. received support from Medical Research Council (MRC) UK (MR/K013386/1). C.Y. is supported by MRC UK (MR/R008167/1) and Cancer Research UK (C63480/A25246).
Robb CT, Goepp M, Rossi AG, Yao C. Non‐steroidal anti‐inflammatory drugs, prostaglandins, and COVID‐19. Br J Pharmacol. 2020;177:4899–4920. 10.1111/bph.15206
REFERENCES
- Aksoy, M. O. , Li, X. , Borenstein, M. , Yi, Y. , & Kelsen, S. G. (1999). Effects of topical corticosteroids on inflammatory mediator‐induced eicosanoid release by human airway epithelial cells. The Journal of Allergy and Clinical Immunology, 103, 1081–1091. [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019a). The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019b). The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Faccenda, E. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Other Protein Targets. British Journal of Pharmacology, 176, S1–S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, R. A. , Gandhi, A. A. , Meng, H. , Yalavarthi, S. , Vreede, A. P. , Estes, S. K. , … Knight, J. S. (2019). Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nature Communications, 10, 1916 10.1038/s41467-019-09801-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici, C. , Di Coro, A. , Ciucci, A. , Chiappa, L. , Castilletti, C. , Martella, V. , … Santoro, M. G. (2006). Indomethacin has a potent antiviral activity against SARS coronavirus. Antivir. Ther. (Lond.), 11, 1021–1030. [PubMed] [Google Scholar]
- Amici, C. , Frazia, S. L. , Brunelli, C. , Balsamo, M. , Angelini, M. , & Santoro, M. G. (2015). Inhibition of viral protein translation by indomethacin in vesicular stomatitis virus infection: Role of eIF2α kinase PKR. Cellular Microbiology, 17, 1391–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki, T. , Frȍsen, J. , Fukuda, M. , Bando, K. , Shioi, G. , Tsuji, K. , … Narumiya, S. (2017). Prostaglandin E2‐EP2‐NF‐κB signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Science Signaling, 10, eaah6037 10.1126/scisignal.aah6037 [DOI] [PubMed] [Google Scholar]
- Aso, H. , Ito, S. , Mori, A. , Morioka, M. , Suganuma, N. , Kondo, M. , … Hasegawa, Y. (2012). Prostaglandin E2 enhances interleukin‐8 production via EP4 receptor in human pulmonary microvascular endothelial cells. American Journal of Physiology. Lung Cellular and Molecular Physiology, 302, L266–L273. [DOI] [PubMed] [Google Scholar]
- Barnes, B. J. , Adrover, J. M. , Baxter‐Stoltzfus, A. , Borczuk, A. , Cools‐Lartigue, J. , Crawford, J. M. , … Egeblad, M. (2020). Targeting potential drivers of COVID‐19: Neutrophil extracellular traps. The Journal of Experimental Medicine, 217, e20200652 10.1084/jem.20200652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, P. J. (2006). How corticosteroids control inflammation: Quintiles Prize Lecture 2005. British Journal of Pharmacology, 148, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, R. C. (2020). COVID‐19 update: Covid‐19‐associated coagulopathy. Journal of Thrombosis and Thrombolysis, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhala, N. , Emberson, J. , Merhi, A. , Abramson, S. , Arber, N. , Baron, J. A. , … Baigent, C . (2013). Vascular and upper gastrointestinal effects of non‐steroidal anti‐inflammatory drugs: Meta‐analyses of individual participant data from randomised trials. Lancet, 382, 769–779. 10.1016/S0140-6736(13)60900-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli, B. , Madhavan, M. V. , Jimenez, D. , Chuich, T. , Dreyfus, I. , Driggin, E. , … Global COVID‐19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function . (2020). COVID‐19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow‐up: JACC state‐of‐the‐art review. Journal of the American College of Cardiology, 75, 2950–2973. 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell, M. A. , Maher, S. A. , Dekkak, B. , Jones, V. , Wong, S. , Brook, P. , & Belvisi, M. G. (2015). Anti‐inflammatory effects of PGE2 in the lung: Role of the EP4 receptor subtype. Thorax, 70, 740–747. 10.1136/thoraxjnl-2014-206592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonam, S. R. , Kaveri, S. V. , Sakuntabhai, A. , Gilardin, L. , & Bayry, J. (2020). Adjunct immunotherapies for the management of severely ill COVID‐19 patients. Cell Rep Med, 1, 100016 10.1016/j.xcrm.2020.100016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno, A. , Albano, G. D. , Siena, L. , Montalbano, A. M. , Riccobono, L. , Anzalone, G. , … Sala, A. (2016). Prostaglandin E2 possesses different potencies in inducing vascular endothelial growth factor and interleukin‐8 production in COPD human lung fibroblasts. Prostaglandins, Leukotrienes and Essential Fatty Acids, 106, 11–18. 10.1016/j.plefa.2016.01.005 [DOI] [PubMed] [Google Scholar]
- Boniface, K. , Bak‐Jensen, K. S. , Li, Y. , Blumenschein, W. M. , McGeachy, M. J. , McClanahan, T. K. , … de Waal Malefyt, R. (2009). Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. The Journal of Experimental Medicine, 206, 535–548. 10.1084/jem.20082293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann, V. , Reichard, U. , Goosmann, C. , Fauler, B. , Uhlemann, Y. , Weiss, D. S. , … Zychlinsky, A. (2004). Neutrophil extracellular traps kill bacteria. Science, 303, 1532–1535. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- Capuano, A. , Scaglione, F. , Berrino, L. , Del Re, M. , Bernardini, R. , Chiamulera, C. , … Racagni, G. (2020). Official Statement of the section of Clinical Pharmacology of Italian Society of Pharmacology on non‐steroidal anti‐inflammatory drugs (NSAIDs) and the increased risk of complications during infections. Pharm Adv, 02 10.36118/pharmadvances.01.2020.03 [DOI] [Google Scholar]
- Castro, V. M. , Ross, R. A. , McBride, S. M. , & Perlis, R. H. (2020). Identifying common pharmacotherapies associated with reduced COVID‐19 morbidity using electronic health records. MedRxiv, 2020(04), 11.20061994 10.1101/2020.04.11.20061994 [DOI] [Google Scholar]
- Channappanavar, R. , Zhao, J. , & Perlman, S. (2014). T cell‐mediated immune response to respiratory coronaviruses. Immunologic Research, 59, 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, B.‐C. , Liao, C.‐C. , Hsu, M.‐J. , Liao, Y.‐T. , Lin, C.‐C. , Sheu, J.‐R. , & Lin, C. H. (2006). Peptidoglycan‐induced IL‐6 production in RAW 264.7 macrophages is mediated by cyclooxygenase‐2, PGE2/PGE4 receptors, protein kinase A, IκB kinase, and NF‐κB. Journal of Immunology, 177, 681–693. 10.4049/jimmunol.177.1.681 [DOI] [PubMed] [Google Scholar]
- Chen, G. , Wu, D. , Guo, W. , Cao, Y. , Huang, D. , Wang, H. , … Ning, Q. (2020). Clinical and immunological features of severe and moderate coronavirus disease 2019. The Journal of Clinical Investigation, 130, 2620–2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Jiang, Q. , Xia, X. , Liu, K. , Yu, Z. , Gong, W. , & Han, J. D. J. (2020). Individual variation of the SARS‐CoV2 receptor ACE2 gene expression and regulation. Preprints, 2020030191 https://www.preprints.org/manuscript/202003.0191/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. H. , Perry, C. J. , Tsui, Y.‐C. , Staron, M. M. , Parish, I. A. , Dominguez, C. X. , … Kaech, S. M. (2015). Prostaglandin E2 and programmed cell death 1 signaling coordinately impair CTL function and survival during chronic viral infection. Nature Medicine, 21, 327–334. 10.1038/nm.3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N. , Zhou, M. , Dong, X. , Qu, J. , Gong, F. , Han, Y. , … Zhang, L. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet, 395, 507–513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, C.‐Y. , Huang, W.‐R. , Chi, P.‐I. , Chiu, H.‐C. , & Liu, H.‐J. (2015). Cell entry of bovine ephemeral fever virus requires activation of Src‐JNK‐AP1 and PI3K‐Akt‐NF‐κB pathways as well as Cox‐2‐mediated PGE2/EP receptor signalling to enhance clathrin‐mediated virus endocytosis. Cellular Microbiology, 17, 967–987. [DOI] [PubMed] [Google Scholar]
- Cho, J.‐S. , Han, I.‐H. , Lee, H. R. , & Lee, H.‐M. (2014). Prostaglandin E2 induces IL‐6 and IL‐8 production by the EP receptors/Akt/NF‐κB pathways in nasal polyp‐derived fibroblasts. Allergy Asthma Immunol Res, 6, 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe, F. , Jaworska, J. , Verway, M. , Tzelepis, F. , Massoud, A. , Gillard, J. , … Divangahi, M. (2014). Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity, 40, 554–568. 10.1016/j.immuni.2014.02.013 [DOI] [PubMed] [Google Scholar]
- Cui, J. , Li, F. , & Shi, Z.‐L. (2019). Origin and evolution of pathogenic coronaviruses. Nature Reviews. Microbiology, 17, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, S. , Chen, S. , Li, X. , Liu, S. , & Wang, F. (2020). Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis, 18, 1421–1424. 10.1111/jth.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, U. N. (2018). Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: A review. Journal of Advanced Research, 11, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, U. N. (2020). Can bioactive lipids inactivate coronavirus (COVID‐19)? Archives of Medical Research, 51, 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J. S. , Lee, H. Y. , Kim, J. , Advani, S. M. , Peng, H.‐L. , Banfield, E. , … Frazier‐Wood, A. C. (2017). Use of non‐steroidal anti‐inflammatory drugs in US adults: Changes over time and by demographic. Open Heart, 4, e000550 10.1136/openhrt-2016-000550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, M. (2020). Covid‐19: Ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ, m1086 10.1136/bmj.m1086 [DOI] [PubMed] [Google Scholar]
- Dehle, F. C. , Mukaro, V. R. , Jurisevic, C. , Moffat, D. , Ahern, J. , Hodge, G. , … Hodge, S. (2013). Defective lung macrophage function in lung cancer ± chronic obstructive pulmonary disease (COPD/emphysema)‐mediated by cancer cell production of PGE2? PLoS ONE, 8, e61573 10.1371/journal.pone.0061573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney, V. B. , & Segel, D. P. (1985). Indomethacin‐induced renal insufficiency: Recurrence on rechallenge. Southern Medical Journal, 78, 1390–1392. [DOI] [PubMed] [Google Scholar]
- Diao, B. , Wang, C. , Tan, Y. , Chen, X. , Liu, Y. , Ning, L. , … Chen, Y. (2020). Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID‐19). Frontiers in Immunology, 11, 827 10.3389/fimmu.2020.00827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz‐Muñoz, M. D. , Osma‐García, I. C. , Cacheiro‐Llaguno, C. , Fresno, M. , & Iñiguez, M. A. (2010). Coordinated up‐regulation of cyclooxygenase‐2 and microsomal prostaglandin E synthase 1 transcription by nuclear factor kappa B and early growth response‐1 in macrophages. Cellular Signalling, 22, 1427–1436. [DOI] [PubMed] [Google Scholar]
- Docherty, A. B. , Harrison, E. M. , Green, C. A. , Hardwick, H. E. , Pius, R. , Norman, L. , … Semple, M. G. (2020). Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ, m1985 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo‐Gonzalez, R. , Martínez‐Colón, G. J. , Smith, A. J. , Smith, C. K. , Ballinger, M. N. , Xia, M. , … Moore, B. B. (2016). Inhibition of neutrophil extracellular trap formation after stem cell transplant by prostaglandin E2 . American Journal of Respiratory and Critical Care Medicine, 193, 186–197. 10.1164/rccm.201501-0161OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris, S. L. , & Peebles, R. S. (2012). PGI2 as a regulator of inflammatory diseases. Mediators of Inflammation, 2012, 926968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward, D. A. , Russell, C. D. , Um, I. H. , Elshani, M. , Armstrong, S. D. , Penrice‐Randal, R. , … Lucas, C. D. (2020). Tissue‐specific tolerance in fatal Covid‐19. medRixv. 10.1101/2020.07.02.20145003 [DOI] [Google Scholar]
- Du, Y. , Kitzmiller, J. A. , Sridharan, A. , Perl, A. K. , Bridges, J. P. , Misra, R. S. , … Potter, S. S. (2017). Lung Gene Expression Analysis (LGEA): An integrative web portal for comprehensive gene expression data analysis in lung development. Thorax, 72, 481–484. 10.1136/thoraxjnl-2016-209598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois, R. N. , Abramson, S. B. , Crofford, L. , Gupta, R. A. , Simon, L. S. , Van De Putte, L. B. , & Lipsky, P. E. (1998). Cyclooxygenase in biology and disease. The FASEB Journal, 12, 1063–1073. 10.1096/fasebj.12.12.1063 [DOI] [PubMed] [Google Scholar]
- Duffin, R. , O'Connor, R. A. , Crittenden, S. , Forster, T. , Yu, C. , Zheng, X. , & Yao, C. (2016). Prostaglandin E2 constrains systemic inflammation through an innate lymphoid cell‐IL‐22 axis. Science, 351, 1333–1338. 10.1126/science.aad9903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eby, J. C. , Gray, M. C. , & Hewlett, E. L. (2014). Cyclic AMP‐mediated suppression of neutrophil extracellular trap formation and apoptosis by the Bordetella pertussis adenylate cyclase toxin. Infection and Immunity, 82, 5256–5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo, Y. , Blinova, K. , Romantseva, T. , Golding, H. , & Zaitseva, M. (2014). Differences in PGE2 production between primary human monocytes and differentiated macrophages: Role of IL‐1β and TRIF/IRF3. PLoS ONE, 9, e98517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius, D. , Neubauer, M. , Mandel, B. , Schütz, C. , Viardot, A. , Vollmer, A. , … Debatin, K. M. (2010). Prostaglandin E2 inhibits IFN‐α secretion and Th1 costimulation by human plasmacytoid dendritic cells via E‐prostanoid 2 and E‐prostanoid 4 receptor engagement. Journal of Immunology, 184, 677–684. 10.4049/jimmunol.0902028 [DOI] [PubMed] [Google Scholar]
- Felton, J. M. , Duffin, R. , Robb, C. T. , Crittenden, S. , Anderton, S. M. , Howie, S. E. M. , … Yao, C. (2018). Facilitation of IL‐22 production from innate lymphoid cells by prostaglandin E2 prevents experimental lung neutrophilic inflammation. Thorax, 73, 1081–1084. 10.1136/thoraxjnl-2017-211097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald, G. A. (2020). Misguided drug advice for COVID‐19. Science, 367, 1434 10.1126/science.abb8034 [DOI] [PubMed] [Google Scholar]
- Fox, S. E. , Akmatbekov, A. , Harbert, J. L. , Li, G. , Quincy Brown, J. , & Vander Heide, R. S. (2020). Pulmonary and cardiac pathology in African American patients with COVID‐19: An autopsy series from New Orleans. The Lancet Respiratory Medicine, S2213260020302435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freites, D. , Leon, L. , Mucientes, A. , Rodriguez‐Rodriguez, L. , Font, J. , Madrid, A. , … Abasolo, L. (2020). Risk factors for hospital admission related to COVID‐19 in inflammatory rheumatic diseases. MedRxiv, 2020(05), 14.20101584. [Google Scholar]
- Friedman, E. A. , Ogletree, M. L. , Haddad, E. V. , & Boutaud, O. (2015). Understanding the role of prostaglandin E2 in regulating human platelet activity in health and disease. Thrombosis Research, 136, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, P. L. , Brown, E. J. Jr. , Gunther, S. , Alexander, R. W. , Barry, W. H. , Mudge, G. H. Jr. , & Grossman, W. (1981). Coronary vasoconstrictor effect of indomethacin in patients with coronary‐artery disease. The New England Journal of Medicine, 305, 1171–1175. 10.1056/NEJM198111123052002 [DOI] [PubMed] [Google Scholar]
- Fuchs, T. A. , Abed, U. , Goosmann, C. , Hurwitz, R. , Schulze, I. , Wahn, V. , … Zychlinsky, A. (2007). Novel cell death program leads to neutrophil extracellular traps. The Journal of Cell Biology, 176, 231–241. 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainaru, G. , Papadopoulos, A. , Tsangaris, I. , Lada, M. , Giamarellos‐Bourboulis, E. J. , & Pistiki, A. (2018). Increases in inflammatory and CD14dim/CD16pos/CD45pos patrolling monocytes in sepsis: Correlation with final outcome. Critical Care, 22, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Rodríguez, L. A. , Tacconelli, S. , & Patrignani, P. (2008). Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti‐inflammatory drugs in the general population. Journal of the American College of Cardiology, 52, 1628–1636. [DOI] [PubMed] [Google Scholar]
- Giamarellos‐Bourboulis, E. J. , Netea, M. G. , Rovina, N. , Akinosoglou, K. , Antoniadou, A. , Antonakos, N. , … Koutsoukou, A. (2020). Complex immune dysregulation in COVID‐19 patients with severe respiratory failure. Cell Host & Microbe, 27, 992–1000.e3. 10.1016/j.chom.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, S. K. , Yao, Y. , Kay, L. J. , Bewley, M. A. , Marriott, H. M. , & Peachell, P. T. (2016). The anti‐inflammatory effects of PGE2 on human lung macrophages are mediated by the EP4 receptor. British Journal of Pharmacology, 173, 3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno, A. , Mestres‐Truyol, J. , Ojeda‐Montes, M. J. , Macip, G. , Saldivar‐Espinoza, B. , Cereto‐Massagué, A. , … Garcia‐Vallvé, S. (2020). Prediction of novel inhibitors of the main protease (M‐pro) of SARS‐CoV‐2 through consensus docking and drug reposition. International Journal of Molecular Sciences, 21, 3793 10.3390/ijms21113793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladding, P. A. , Webster, M. W. I. , Farrell, H. B. , Zeng, I. S. L. , Park, R. , & Ruijne, N. (2008). The antiplatelet effect of six non‐steroidal anti‐inflammatory drugs and their pharmacodynamic interaction with aspirin in healthy volunteers. The American Journal of Cardiology, 101, 1060–1063. [DOI] [PubMed] [Google Scholar]
- Gomeni, R. , Xu, T. , Gao, X. , & Bressolle‐Gomeni, F. (2020). Model based approach for estimating the dosage regimen of indomethacin a potential antiviral treatment of patients infected with SARS CoV‐2. Journal of Pharmacokinetics and Pharmacodynamics, 47, 189–198. 10.1007/s10928-020-09690-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, D. E. , Jang, G. M. , Bouhaddou, M. , Xu, J. , Obernier, K. , White, K. M. , … Krogan, N. J. (2020). A SARS‐CoV‐2 protein interaction map reveals targets for drug repurposing. Nature, 583, 459–468. 10.1038/s41586-020-2286-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia‐Figueira, S. , Karimpour, M. , Bosson, J. A. , Blomberg, A. , Unosson, J. , Pourazar, J. , … Nording, M. L. (2017). Mass spectrometry profiling of oxylipins, endocannabinoids, and N‐acylethanolamines in human lung lavage fluids reveals responsiveness of prostaglandin E2 and associated lipid metabolites to biodiesel exhaust exposure. Analytical and Bioanalytical Chemistry, 409, 2967–2980. 10.1007/s00216-017-0243-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, S. , Tilly, P. , Hentsch, D. , Vonesch, J.‐L. , & Fabre, J.‐E. (2007). Vascular wall–produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors. The Journal of Experimental Medicine, 204, 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser, T. , Fries, S. , & FitzGerald, G. A. (2006). Biological basis for the cardiovascular consequences of COX‐2 inhibition: Therapeutic challenges and opportunities. The Journal of Clinical Investigation, 116, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W. , Ni, Z. , Hu, Y. , Liang, W. , Ou, C. , He, J. , … China Medical Treatment Expert Group for Covid‐19 . (2020). Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine, 382, 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson, K. , Junevik, K. , Werlenius, O. , Holmgren, S. , Karlsson‐Parra, A. , & Andersson, P.‐O. (2011). Tumour‐loaded α‐type 1‐polarized dendritic cells from patients with chronic lymphocytic leukaemia produce a superior NK‐, NKT‐ and CD8+ T cell‐attracting chemokine profile. Scandinavian Journal of Immunology, 74, 318–326. [DOI] [PubMed] [Google Scholar]
- Hadjadj, J. , Yatim, N. , Barnabei, L. , Corneau, A. , Boussier, J. , Pere, H. , … Terrier, B. (2020). Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid‐19 patients. medRixv. 10.1101/2020.04.19.20068015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming, I. , Timens, W. , Bulthuis, M. L. C. , Lely, A. T. , Navis, G. J. , & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology, 203, 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, K. , Graham, B. S. , Geraci, M. W. , FitzGerald, G. A. , Egan, K. , Zhou, W. , … Peebles, R. S. Jr. (2004). Signaling through the prostaglandin I2 receptor IP protects against respiratory syncytial virus‐induced illness. Journal of Virology, 78, 10303–10309. 10.1128/JVI.78.19.10303-10309.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkey, C. J. , & Truelove, S. C. (1981). Effect of prednisolone on prostaglandin synthesis by rectal mucosa in ulcerative colitis: Investigation by laminar flow bioassay and radioimmunoassay. Gut, 22, 190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz, A. L. , Bender, A. T. , Smith, K. C. , Gilchrist, M. , Amieux, P. S. , Aderem, A. , & Beavo, J. A. (2009). Elevated cyclic AMP and PDE4 inhibition induce chemokine expression in human monocyte‐derived macrophages. Proceedings. National Academy of Sciences. United States of America, 106, 21978–21983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson, R. M. , Williams, J. A. , & Shacter, E. (1996). Elevated interleukin 6 is induced by prostaglandin E2 in a murine model of inflammation: Possible role of cyclooxygenase‐2. Proceedings. National Academy of Sciences. United States of America, 93, 4885–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , … Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181, 271–280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, D. , Ma, X. , Kundu, N. , & Fulton, A. (2011). Prostaglandin E2 (PGE2) suppresses natural killer cell function primarily through the PGE2 receptor EP4. Cancer Immunology, Immunotherapy, 60, 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, T. , Segi‐Nishida, E. , Miyachi, Y. , & Narumiya, S. (2006). Prostacyclin‐IP signaling and prostaglandin E2‐EP2/EP4 signaling both mediate joint inflammation in mouse collagen‐induced arthritis. The Journal of Experimental Medicine, 203, 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, W. , Chen, Y. , You, K. , Tan, S. , Wu, F. , Tao, J. , … Li, Y. (2020). Celebrex adjuvant therapy on COVID‐19: An experimental study. MedRxiv, 2020, 05.05.20077610. [Google Scholar]
- Horby, P. , Lim, W. S. , Emberson, J. , Mafham, M. , Bell, J. , Linsell, L. , … Landray, M. J. (2020). Effect of dexamethasone in hospitalized patients with COVID‐19: Preliminary report. medRxiv, 2020.06.22.20137273 10.1101/2020.06.22.20137273 [DOI] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395, 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh, K. , Ji, W. , Kang, M. , Hong, J. , Bae, G. H. , Lee, R. , … Jung, J. (2020). Association of previous medications with the risk of COVID‐19: A nationwide claims‐based study from South Korea. MedRxiv, 2020(05), 04.20089904. [Google Scholar]
- Hung, I. F. N. , To, K. K. W. , Chan, J. F. W. , Cheng, V. C. C. , Liu, K. S. H. , Tam, A. , … Yuen, K. Y. (2017). Efficacy of clarithromycin‐naproxen‐oseltamivir combination in the treatment of patients hospitalized for influenza A (H3N2) infection: An open‐label randomized, controlled, phase IIb/III trial. Chest, 151, 1069–1080. 10.1016/j.chest.2016.11.012 [DOI] [PubMed] [Google Scholar]
- Imai, Y. , Kuba, K. , & Penninger, J. M. (2008). The discovery of angiotensin‐converting enzyme 2 and its role in acute lung injury in mice. Experimental Physiology, 93, 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, Y. , Kuba, K. , Rao, S. , Huan, Y. , Guo, F. , Guan, B. , … Penninger, J. M. (2005). Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature, 436, 112–116. 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, H. , Takamori, M. , Shimoyama, Y. , Ishibashi, H. , Yamamoto, S. , & Koshihara, Y. (2002). Regulation by PGE2 of the production of interleukin‐6, macrophage colony stimulating factor, and vascular endothelial growth factor in human synovial fibroblasts. British Journal of Pharmacology, 136, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irion, E. , & Blombäck, M. (1969). Prostaglandins in platelet aggregation. Scandinavian Journal of Clinical and Laboratory Investigation, 24, 141–144. [DOI] [PubMed] [Google Scholar]
- Jia, H. P. , Look, D. C. , Shi, L. , Hickey, M. , Pewe, L. , Netland, J. , … McCray, P. B. Jr. (2005). ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. JVI, 79, 14614–14621. 10.1128/JVI.79.23.14614-14621.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, H. S. , Gu, J. , Kim, J.‐E. , Nam, Y. , Song, J. W. , & Kim, H. K. (2019). Cancer cell‐induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PLoS ONE, 14, e0216055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliński, P. , Hilkens, C. M. , Snijders, A. , Snijdewint, F. G. , & Kapsenberg, M. L. (1997). IL‐12‐deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. Journal of Immunology, 159, 28–35. [PubMed] [Google Scholar]
- Kaplan, M. J. , & Radic, M. (2012). Neutrophil extracellular traps (NETs): Double‐edged swords of innate immunity. Journal of Immunology, 189, 2689–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi, E. , Shiota, M. , Yokomizo, A. , Inokuchi, J. , Uchiumi, T. , & Naito, S. (2014). EP2 signaling mediates suppressive effects of celecoxib on androgen receptor expression and cell proliferation in prostate cancer. Prostate Cancer and Prostatic Diseases, 17, 10–17. [DOI] [PubMed] [Google Scholar]
- Khan, S. , Siddique, R. , Shereen, M. A. , Ali, A. , Liu, J. , Bai, Q. … Xue, M. (2020). Emergence of a novel coronavirus, severe acute respiratory syndrome coronavirus 2: Biology and therapeutic options. Journal of Clinical Microbiology, 58, e00187–e00120. /jcm/58/5/JCM.00187‐20.atom [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilinç, E. , & Kilinç, Y. B. (2020). Mast cell stabilizers as a supportive therapy can contribute to alleviate fatal inflammatory responses and severity of pulmonary complications in COVID‐19 infection. Anadolu Kliniği Tıp Bilimleri Dergisi, 25, 111–118. [Google Scholar]
- Klok, F. A. , Kruip, M. J. H. A. , van der Meer, N. J. M. , Arbous, M. S. , Gommers, D. A. M. P. J. , Kant, K. M. , … Endeman, H. (2020). Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thrombosis Research, 191, 145–147. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, G. , Schönfeld, N. , Jost, K. , Atkinson, A. , Schulzke, S. M. , Pfister, M. , & Datta, A. N. (2020). Caffeine preserves quiet sleep in preterm neonates. Pharmacology Research & Perspectives, 8, e00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari, P. , Pestana, R. , Mesraoua, R. , Elchaki, R. , Khan, K. M. F. , Dannenberg, A. J. , & Falcone, D. J. (2014). IL‐6‐mediated induction of matrix metalloproteinase‐9 is modulated by JAK‐dependent IL‐10 expression in macrophages. Journal of Immunology, 192, 349–357. 10.4049/jimmunol.1301906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba, K. , Imai, Y. , Rao, S. , Gao, H. , Guo, F. , Guan, B. , … Penninger, J. M. (2005). A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nature Medicine, 11, 875–879. 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba, K. , Imai, Y. , Rao, S. , Jiang, C. , & Penninger, J. M. (2006). Lessons from SARS: Control of acute lung failure by the SARS receptor ACE2. Journal of Molecular Medicine (Berlin, Germany), 84, 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumei, S. , Yuhki, K.‐I. , Kojima, F. , Kashiwagi, H. , Imamichi, Y. , Okumura, T. , … Ushikubi, F. (2018). Prostaglandin I2 suppresses the development of diet‐induced nonalcoholic steatohepatitis in mice. The FASEB Journal, 32, 2354–2365. 10.1096/fj.201700590R [DOI] [PubMed] [Google Scholar]
- Lachowicz‐Scroggins, M. E. , Dunican, E. M. , Charbit, A. R. , Raymond, W. , Looney, M. R. , Peters, M. C. , … Fahy, J. V. (2019). Extracellular DNA, neutrophil extracellular traps, and inflammasome activation in severe asthma. American Journal of Respiratory and Critical Care Medicine, 199, 1076–1085. 10.1164/rccm.201810-1869OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers, M. M. , Beumer, J. , van der Vaart, J. , Knoops, K. , Puschhof, J. , Breugem, T. I. , … Clevers, H. (2020). SARS‐CoV‐2 productively infects human gut enterocytes. Science, 369, 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer, S. A. , Grantz, K. H. , Bi, Q. , Jones, F. K. , Zheng, Q. , Meredith, H. R. , … Lessler, J. (2020). The incubation period of coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: Estimation and application. Annals of Internal Medicine, 172, 577–582. 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourgeois, M. , Ferroni, A. , Leruez‐Ville, M. , Varon, E. , Thumerelle, C. , Brémont, F. , … Guillemot, D. (2016). Nonsteroidal anti‐inflammatory drug without antibiotics for acute viral infection increases the empyema risk in children: A matched case‐control study. The Journal of Pediatrics, 175, 47–53.e3. 10.1016/j.jpeds.2016.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C.‐H. , Chen, R.‐F. , Liu, J.‐W. , Yeh, W.‐T. , Chang, J.‐C. , Liu, P.‐M. , … Yang, K. D. (2004). Altered p38 mitogen‐activated protein kinase expression in different leukocytes with increment of immunosuppressive mediators in patients with severe acute respiratory syndrome. Journal of Immunology, 172, 7841–7847. 10.4049/jimmunol.172.12.7841 [DOI] [PubMed] [Google Scholar]
- Lee, J. , Aoki, T. , Thumkeo, D. , Siriwach, R. , Yao, C. , & Narumiya, S. (2019). T cell‐intrinsic prostaglandin E2‐EP2/EP4 signaling is critical in pathogenic TH17 cell‐driven inflammation. The Journal of Allergy and Clinical Immunology, 143, 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard‐Lorant, I. , Delabranche, X. , Severac, F. , Helms, J. , Pauzet, C. , Collange, O. , … Ohana, M. (2020). Acute pulmonary embolism in COVID‐19 patients on CT angiography and relationship to D‐dimer levels. Radiology, 201561 10.1148/radiol.2020201561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi, M. , Thachil, J. , Iba, T. , & Levy, J. H. (2020). Coagulation abnormalities and thrombosis in patients with COVID‐19. The Lancet Haematology, 7, e438–e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Guan, K. , Zhou, Y. , Wu, J. , Wang, Y. , & Wang, W. (2019). Prostaglandin E2 signal inhibits T regulatory cell differentiation during allergic rhinitis inflammation through EP4 receptor. World Allergy Organization Journal, 12, 100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T. , Qi, J. , & Cowley, E. A. (2011). Activation of the EP4 prostanoid receptor induces prostaglandin E2 and pro‐inflammatory cytokine production in human airway epithelial cells. Pulmonary Pharmacology & Therapeutics, 24, 42–48. [DOI] [PubMed] [Google Scholar]
- Li, W. , Wang, W. , Zuo, R. , Liu, C. , Shu, Q. , Ying, H. , & Sun, K. (2017). Induction of pro‐inflammatory genes by serum amyloid A1 in human amnion fibroblasts. Scientific Reports, 7, 693 10.1038/s41598-017-00782-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Lei, D. , Swindell, W. R. , Xia, W. , Weng, S. , Fu, J. , … Fisher, G. J. (2015). Age‐associated increase in skin fibroblast‐derived prostaglandin E2 contributes to reduced collagen levels in elderly human skin. The Journal of Investigative Dermatology, 135, 2181–2188. 10.1038/jid.2015.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, M. , Liu, Y. , Yuan, J. , Wen, Y. , Xu, G. , Zhao, J. , … Zhang, Z. (2020). Single‐cell landscape of bronchoalveolar immune cells in patients with COVID‐19. Nature Medicine, 26, 842–844. [DOI] [PubMed] [Google Scholar]
- Liberale, L. , Holy, E. W. , Akhmedov, A. , Bonetti, N. R. , Nietlispach, F. , Matter, C. M. , … Camici, G. G. (2019). Interleukin‐1β mediates arterial thrombus formation via NET‐associated tissue factor. Journal of Clinical Medicine, 8 10.3390/jcm8122072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi, G. , & Favaloro, E. J. (2020). D‐dimer is associated with severity of coronavirus disease 2019: A pooled analysis. Thrombosis and Haemostasis, 120, 876–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little, P. (2020). Non‐steroidal anti‐inflammatory drugs and covid‐19. BMJ, m1185. [DOI] [PubMed] [Google Scholar]
- Little, P. , Moore, M. , Kelly, J. , Williamson, I. , Leydon, G. , McDermott, L. , … PIPS investigators . (2013). Ibuprofen, paracetamol, and steam for patients with respiratory tract infections in primary care: Pragmatic randomised factorial trial. BMJ, 347, f6041–f6041. 10.1136/bmj.f6041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D. X. , Liang, J. Q. , & Fung, T. S. (2020). Human coronavirus‐229E, ‐OC43, ‐NL63, and ‐HKU1. Reference Module in Life Sciences.. 10.1016/B978-0-12-809633-8.21501-X [DOI] [Google Scholar]
- Liu, J. , Li, S. , Liu, J. , Liang, B. , Wang, X. , Wang, H. , … Zheng, X. (2020). Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. eBioMedicine, 55, 102763 10.1016/j.ebiom.2020.102763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, R. , Zhao, X. , Li, J. , Niu, P. , Yang, B. , Wu, H. , … Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet, 395, 565–574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundequist, A. , Nallamshetty, S. N. , Xing, W. , Feng, C. , Laidlaw, T. M. , Uematsu, S. , … Boyce, J. A. (2010). Prostaglandin E2 exerts homeostatic regulation of pulmonary vascular remodeling in allergic airway inflammation. Journal of Immunology, 184, 433–441. 10.4049/jimmunol.0902835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre, D. E. , & Gordon, J. L. (1975). Calcium‐dependent stimulation of platelet aggregation by PGE2 . Nature, 258, 337–339. [DOI] [PubMed] [Google Scholar]
- Malle, E. , Bollmann, A. , Steinmetz, A. , Gemsa, D. , Leis, H. J. , & Sattler, W. (1997). Serum amyloid A (SAA) protein enhances formation of cyclooxygenase metabolites of activated human monocytes. FEBS Letters, 419, 215–219. [DOI] [PubMed] [Google Scholar]
- Martínez‐Colón, G. J. , Taylor, Q. M. , Wilke, C. A. , Podsiad, A. B. , & Moore, B. B. (2018). Elevated prostaglandin E2 post‐bone marrow transplant mediates interleukin‐1β‐related lung injury. Mucosal Immunology, 11, 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, J. T. , Torres, V. E. , Romero, J. C. , Wochos, D. N. , & Velosa, J. A. (1982). Acute intrinsic renal failure induced by indomethacin: Role of prostaglandin synthetase inhibition. Mayo Clinic Proceedings, 57, 289–296. [PubMed] [Google Scholar]
- Mehta, P. , McAuley, D. F. , Brown, M. , Sanchez, E. , Tattersall, R. S. , Manson, J. J. , & HLH Across Speciality Collaboration, UK . (2020). COVID‐19: Consider cytokine storm syndromes and immunosuppression. Lancet, 395, 1033–1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad, M. , & Martin, J. C. (2020). Pathological inflammation in patients with COVID‐19: A key role for monocytes and macrophages. Nature Reviews. Immunology, 20, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, J. , Lu, X. , Hu, Y. , Piao, C. , Wu, X. , Liu, X. , … Liu, J. (2017). Prostaglandin E2 and PD‐1 mediated inhibition of antitumor CTL responses in the human tumor microenvironment. Oncotarget, 8, 89802–89810. 10.18632/oncotarget.21155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef, J. , Soeiro, T. , & Jonville‐Béra, A.‐P. (2020). Non‐steroidal anti‐inflammatory drugs, pharmacology, and COVID‐19 infection. Thérapie. 10.1016/j.therap.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton, E. A. , He, X.‐Y. , Denorme, F. , Campbell, R. A. , Ng, D. , Salvatore, S. P. , … Yost, C. C. (2020). Neutrophil extracellular traps (NETs) contribute to immunothrombosis in COVID‐19 acute respiratory distress syndrome. Blood. 10.1182/blood.2020007008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer, L. , Moreau, F. , MacDonald, J. A. , & Chadee, K. (2016). NLRP3 inflammasome inhibition is disrupted in a group of auto‐inflammatory disease CAPS mutations. Nature Immunology, 17, 1176–1186. [DOI] [PubMed] [Google Scholar]
- Mozzini, C. , & Girelli, D. (2020). The role of neutrophil extracellular traps in Covid‐19: Only an hypothesis or a potential new field of research? Thrombosis Research, 191, 26–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, C. , Hardt, M. , Schwudke, D. , Neuman, B. W. , Pleschka, S. , & Ziebuhr, J. (2018). Inhibition of cytosolic phospholipase A2α impairs an early step of coronavirus replication in cell culture. Journal of Virology, 92 10.1128/JVI.01463-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, S. , Honda, T. , Sakata, D. , Egawa, G. , Tanizaki, H. , Otsuka, A. , … Kabashima, K. (2010). Prostaglandin I2‐IP signaling promotes Th1 differentiation in a mouse model of contact hypersensitivity. Journal of Immunology, 184, 5595–5603. 10.4049/jimmunol.0903260 [DOI] [PubMed] [Google Scholar]
- Nakanishi, M. , & Rosenberg, D. W. (2013). Multifaceted roles of PGE2 in inflammation and cancer. Seminars in Immunopathology, 35, 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata, R. , Nakamura, Y. , Hosomi, S. , Okuda, H. , Nishida, Y. , Sugita, N. , … Fujiwara, Y. (2020). Slco2a1 deficiency exacerbates experimental colitis via inflammasome activation in macrophages: A possible mechanism of chronic enteropathy associated with SLCO2A1 gene. Scientific Reports, 10, 4883 10.1038/s41598-020-61775-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya, S. , & FitzGerald, G. A. (2001). Genetic and pharmacological analysis of prostanoid receptor function. The Journal of Clinical Investigation, 108, 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuschäfer‐Rube, F. , Pathe‐Neuschäfer‐Rube, A. , Hippenstiel, S. , Kracht, M. , & Püschel, G. P. (2013). NF‐κB‐dependent IL‐8 induction by prostaglandin E2 receptors EP1 and EP4. British Journal of Pharmacology, 168, 704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton, R. , Seybold, J. , Kuitert, L. M. E. , Bergmann, M. , & Barnes, P. J. (1998). Repression of cyclooxygenase‐2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and post‐transcriptional mechanisms involving loss of polyadenylated mRNA. The Journal of Biological Chemistry, 273, 32312–32321. [DOI] [PubMed] [Google Scholar]
- Nieto‐Torres, J. L. , Verdiá‐Báguena, C. , Jimenez‐Guardeño, J. M. , Regla‐Nava, J. A. , Castaño‐Rodriguez, C. , Fernandez‐Delgado, R. , … Enjuanes, L. (2015). Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology, 485, 330–339. 10.1016/j.virol.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogushi, F. , Ozaki, T. , Kawano, T. , & Yasuoka, S. (1987). PGE2 and PGF2α content in bronchoalveolar lavage fluid obtained from patients with eosinophilic pneumonia. Chest, 91, 204–206. [DOI] [PubMed] [Google Scholar]
- Oxley, T. J. , Mocco, J. , Majidi, S. , Kellner, C. P. , Shoirah, H. , Singh, I. P. , … Fifi, J. T. (2020). Large‐vessel stroke as a presenting feature of Covid‐19 in the young. The New England Journal of Medicine, 382, e60 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, A. , & Iwasaki, A. (2020). Type I and type III interferons—Induction, signaling, evasion, and application to combat COVID‐19. Cell Host & Microbe, 27, 870–878. 10.1016/j.chom.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, A. , Lee, Y. , Kim, M. S. , Kang, Y. J. , Park, Y.‐J. , Jung, H. , … Yoon, S. R. (2018). Prostaglandin E2 secreted by thyroid cancer cells contributes to immune escape through the suppression of natural killer (NK) cell cytotoxicity and NK cell differentiation. Frontiers in Immunology, 9, 1859 10.3389/fimmu.2018.01859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrignani, P. , & Patrono, C. (2015). Cyclooxygenase inhibitors: From pharmacology to clinical read‐outs. Biochimica et Biophysica Acta (BBA) ‐ Molecular and Cell Biology of Lipids, 1851, 422–432. [DOI] [PubMed] [Google Scholar]
- Paulissen, S. M. J. , van Hamburg, J. P. , Davelaar, N. , Asmawidjaja, P. S. , Hazes, J. M. W. , & Lubberts, E. (2013). Synovial fibroblasts directly induce Th17 pathogenicity via the cyclooxygenase/prostaglandin E2 pathway, independent of IL‐23. Journal of Immunology, 191, 1364–1372. [DOI] [PubMed] [Google Scholar]
- van der Pouw Kraan, T. C. , Boeije, L. C. , Smeenk, R. J. , Wijdenes, J. , & Aarden, L. A. (1995). Prostaglandin‐E2 is a potent inhibitor of human interleukin 12 production. The Journal of Experimental Medicine, 181, 775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Z. , Travanty, E. A. , Oko, L. , Edeen, K. , Berglund, A. , Wang, J. , … Mason, R. J. (2013). Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome–coronavirus. American Journal of Respiratory Cell and Molecular Biology, 48, 742–748. 10.1165/rcmb.2012-0339OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, W. , Wang, C. , Chen, B. , Zhang, F. , Liu, Y. , Lu, Q. , … Yin, X. (2015). Ibuprofen attenuates cardiac fibrosis in streptozotocin‐induced diabetic rats. Cardiology, 131, 97–106. 10.1159/000375362 [DOI] [PubMed] [Google Scholar]
- Qin, C. , Zhou, L. , Hu, Z. , Zhang, S. , Yang, S. , Tao, Y. , … Tian, D. S. (2020). Dysregulation of immune response in patients with COVID‐19 in Wuhan. China. Clin. Infect. Dis.. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, G. , Zheng, G. , Ge, M. , Huang, L. , Tong, H. , Chen, P. , … Xu, J. (2017). Adipose‐derived mesenchymal stem cells modulate CD14++CD16+ expression on monocytes from sepsis patients in vitro via prostaglandin E2 . Stem Cell Research & Therapy, 8, 97 10.1186/s13287-017-0546-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabausch, K. , Bretschneider, E. , Sarbia, M. , Meyer‐Kirchrath, J. , Censarek, P. , Pape, R. , … Weber, A. A. (2005). Regulation of thrombomodulin expression in human vascular smooth muscle cells by COX‐2–derived prostaglandins. Circulation Research, 96, e1–e6. 10.1161/01.RES.0000153150.27690.f2 [DOI] [PubMed] [Google Scholar]
- Raj, V. S. , Mou, H. , Smits, S. L. , Dekkers, D. H. W. , Müller, M. A. , Dijkman, R. , … Haagmans, B. L. (2013). Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus‐EMC. Nature, 495, 251–254. 10.1038/nature12005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri, N. , Douglas, R. S. , & Smith, T. J. (2010). PGE2 induces IL‐6 in orbital fibroblasts through EP2 receptors and increased gene promoter activity: Implications to thyroid‐associated ophthalmopathy. PLoS ONE, 5, e15296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentsch, C. T. , Kidwai‐Khan, F. , Tate, J. P. , Park, L. S. , King, J. T. , Skanderson, M. , … Re, V. L. (2020). Covid‐19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54–75 years. MedRxiv, 2020(04), 09.20059964. [Google Scholar]
- Ricciotti, E. , & FitzGerald, G. A. (2011). Prostaglandins and inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 31, 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb, C. T. , Dyrynda, E. A. , Gray, R. D. , Rossi, A. G. , & Smith, V. J. (2014). Invertebrate extracellular phagocyte traps show that chromatin is an ancient defence weapon. Nature Communications, 5, 4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb, C. T. , McSorley, H. J. , Lee, J. , Aoki, T. , Yu, C. , Crittenden, S. , … Yao, C. (2018). Prostaglandin E2 stimulates adaptive IL‐22 production and promotes allergic contact dermatitis. The Journal of Allergy and Clinical Immunology, 141, 152–162. 10.1016/j.jaci.2017.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajiki, Y. , Konnai, S. , Okagawa, T. , Nishimori, A. , Maekawa, N. , Goto, S. , … Ohashi, K. (2019). Prostaglandin E2‐induced immune exhaustion and enhancement of antiviral effects by anti‐PD‐L1 antibody combined with COX‐2 inhibitor in bovine leukemia virus infection. Journal of Immunology, 203, 1313–1324. 10.4049/jimmunol.1900342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander, W. J. , O'Neill, H. G. , & Pohl, C. H. (2017). Prostaglandin E2 as a modulator of viral infections. Frontiers in Physiology, 8, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavon, V. , Duchez, S. , Branchtein, M. , How‐Kit, A. , Cassius, C. , Daunay, A. , … le Buanec, H. (2019). Microenvironment tailors nTreg structure and function. Proceedings. National Academy of Sciences. United States of America, 116, 6298–6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, M. , Christiansen, C. F. , Horváth‐Puhó, E. , Glynn, R. J. , Rothman, K. J. , & Sørensen, H. T. (2011). Non‐steroidal anti‐inflammatory drug use and risk of venous thromboembolism. Journal of Thrombosis and Haemostasis, 9, 1326–1333. [DOI] [PubMed] [Google Scholar]
- Shen, B. , Yi, X. , Sun, Y. , Bi, X. , Du, J. , Zhang, C. , … Guo, T. (2020). Proteomic and metabolomic characterization of COVID‐19 patient sera. Cell, 182, p59–72.e15 10.1016/j.cell.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppe, A. E. F. , Kummari, E. , Walker, A. , Richards, A. , Hui, W. W. , Lee, J. H. , … Edelmann, M. J. (2018). PGE2 augments inflammasome activation and M1 polarization in macrophages infected with Salmonella Typhimurium and Yersinia enterocolitica . Frontiers in Microbiology, 9, 2447 10.3389/fmicb.2018.02447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, C.‐S. , Nabar, N. R. , Huang, N.‐N. , & Kehrl, J. H. (2019). SARS‐coronavirus open reading frame‐8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov, 5, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, H. , Zuo, Y. , Yalavarthi, S. , Gockman, K. , Zuo, M. , Madison, J. A. , … Kanthi, Y. (2020). Neutrophil calprotectin identifies severe pulmonary disease in COVID‐19. MedRxiv. 10.1101/2020.05.06.20093070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Tan, M. , Chen, X. , Liu, Y. , Huang, J. , Ou, J. , & Deng, X . (2020). Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. MedRxiv. 10.1101/2020.03.12.20034736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishikura, K. , Horiuchi, T. , Sakata, N. , Trinh, D.‐A. , Shirakawa, R. , Kimura, T. , … Horiuchi, H. (2016). Prostaglandin E2 inhibits neutrophil extracellular trap formation through production of cyclic AMP. British Journal of Pharmacology, 173, 319–331. 10.1111/bph.13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu, K.‐L. , Yuen, K.‐S. , Castaño‐Rodriguez, C. , Ye, Z.‐W. , Yeung, M.‐L. , Fung, S.‐Y. , … Jin, D.‐Y. (2019). Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3‐dependent ubiquitination of ASC. The FASEB Journal, 33, 8865–8877. 10.1096/fj.201802418R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens, S. P. , van de Veerdonk, F. L. , Joosten, L. A. B. , Jacobs, L. , Jansen, T. , Williams, D. L. , … Netea, M. G. (2011). The classical CD14++CD16− monocytes, but not the patrolling CD14+CD16+ monocytes, promote Th17 responses to Candida albicans . European Journal of Immunology, 41, 2915–2924. 10.1002/eji.201141418 [DOI] [PubMed] [Google Scholar]
- Smeitink, J. , Jiang, X. , Pecheritsyna, S. , Renkema, H. , van Maanen, R. , & Beyrath, J. (2020). Hypothesis: mPGES‐1‐derived prostaglandin E2, a so far missing link in COVID‐19 pathophysiology? Preprints, 2020040180 10.20944/preprints202004.0180.v1 [DOI] [Google Scholar]
- Smith, J. B. , Silver, M. J. , Ingerman, C. M. , & Kocsis, J. J. (1974). Prostaglandin D2 inhibits the aggregation of human platelets. Thrombosis Research, 5, 291–299. [DOI] [PubMed] [Google Scholar]
- Sokolowska, M. , Chen, L.‐Y. , Liu, Y. , Martinez‐Anton, A. , Qi, H.‐Y. , Logun, C. , … Shelhamer, J. H. (2015). Prostaglandin E2 inhibits NLRP3 inflammasome activation through EP4 receptor and intracellular cyclic AMP in human macrophages. Journal of Immunology, 194, 5472–5487. 10.4049/jimmunol.1401343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman, L. J. , Bellamy, R. , & Garner, P. (2006). SARS: Systematic review of treatment effects. PLoS Medicine, 3, e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, L. , Wang, Q. , Zhang, D. , Ding, J. , Huang, Q. , Tang, Y.‐Q. , … Miao, H. (2020). Lymphopenia predicts disease severity of COVID‐19: A descriptive and predictive study. MedRxiv. 10.1101/2020.03.01.20029074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, N. , Li, D. , Wang, X. , & Sun, Z. (2020). Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis, 18, 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay, M. Z. , Poh, C. M. , Rénia, L. , MacAry, P. A. , & Ng, L. F. P. (2020). The trinity of COVID‐19: Immunity, inflammation and intervention. Nature Reviews. Immunology, 20, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theobald, S. J. , Simonis, A. , Kreer, C. , Zehner, M. , Fischer, J. , Albert, M.‐C. , … Rybniker, J. (2020). The SARS‐CoV‐2 spike protein primes inflammasome‐mediated interleukin‐1‐beta secretion in COVID‐19 patient‐derived macrophages (Preprint). Research Square. 10.21203/rs.3.rs-30407/v1 [DOI] [Google Scholar]
- Thierry, A. , and Roch, B. (2020). NETs by‐products and extracellular DNA may play a key role in COVID‐19 pathogenesis: Incidence on patient monitoring and therapy. Preprints, 2020040238 10.20944/preprints202004.0238.v1 [DOI] [Google Scholar]
- Thierry, A. R. (2020). Does the newly observed inflammatory syndrome in children demonstrate a link between uncontrolled neutrophil extracellular traps formation and COVID‐19? Pediatric Research. 10.1038/s41390-020-0996-1 [DOI] [PubMed] [Google Scholar]
- Tian, S. , Xiong, Y. , Liu, H. , Niu, L. , Guo, J. , Liao, M. , & Xiao, S. Y. (2020). Pathological study of the 2019 novel coronavirus disease (COVID‐19) through postmortem core biopsies. Modern Pathology, 33, 1007–1014. 10.1038/s41379-020-0536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki, S. , Reiss, S. , Goleniewska, K. , Moore, M. L. , FitzGerald, G. , & Peebles, R. S. (2013). Prostaglandin I2 receptor (IP) signaling inhibits lung type I interferon expression by RSV infection. Journal of Allergy and Clinical Immunology, 131, AB191. [Google Scholar]
- Tomar, B. , Anders, H.‐J. , Desai, J. , & Mulay, S. R. (2020). Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID‐19. Cell, 9, 1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truchetet, M.‐E. , Allanore, Y. , Montanari, E. , Chizzolini, C. , & Brembilla, N. C. (2012). Prostaglandin I2 analogues enhance already exuberant Th17 cell responses in systemic sclerosis. Annals of the Rheumatic Diseases, 71, 2044–2050. [DOI] [PubMed] [Google Scholar]
- Van Elssen, C. H. M. J. , Vanderlocht, J. , Oth, T. , Senden‐Gijsbers, B. L. M. G. , Germeraad, W. T. V. , & Bos, G. M. J. (2011). Inflammation‐restraining effects of prostaglandin E2 on natural killer‐dendritic cell (NK‐DC) interaction are imprinted during DC maturation. Blood, 118, 2473–2482. [DOI] [PubMed] [Google Scholar]
- Vancheri, C. , Mastruzzo, C. , Sortino, M. A. , & Crimi, N. (2004). The lung as a privileged site for the beneficial actions of PGE2 . Trends in Immunology, 25, 40–46. [DOI] [PubMed] [Google Scholar]
- Vijay, R. , Fehr, A. R. , Janowski, A. M. , Athmer, J. , Wheeler, D. L. , Grunewald, M. , … Perlman, S. (2017). Virus‐induced inflammasome activation is suppressed by prostaglandin D2/DP1 signaling. PNAS, 114, E5444–E5453. 10.1073/pnas.1704099114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay, R. , Hua, X. , Meyerholz, D. K. , Miki, Y. , Yamamoto, K. , Gelb, M. , … Perlman, S. (2015). Critical role of phospholipase A2 group IID in age‐related susceptibility to severe acute respiratory syndrome–CoV infection. The Journal of Experimental Medicine, 212, 1851–1868. 10.1084/jem.20150632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiriot, G. , Philippot, Q. , Elabbadi, A. , Elbim, C. , Chalumeau, M. , & Fartoukh, M. (2019). Risks related to the use of non‐steroidal anti‐inflammatory drugs in community‐acquired pneumonia in adult and pediatric patients. Journal of Clinical Medicine, 8, 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls, A. C. , Park, Y.‐J. , Tortorici, M. A. , Wall, A. , McGuire, A. T. , & Veesler, D. (2020). Structure, function and antigenicity of the SARS‐CoV‐2 spike glycoprotein (Biochemistry). BioRevix. 10.1101/2020.02.19.956581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B. , Li, R. , Lu, Z. , & Huang, Y. (2020). Does comorbidity increase the risk of patients with COVID‐19: Evidence from meta‐analysis. Aging. 10.18632/aging.103000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , & DuBois, R. N. (2018). Role of prostanoids in gastrointestinal cancer. The Journal of Clinical Investigation, 128, 2732–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, W. , Su, W. , Tang, H. , Le, W. , Zhang, X. , Zheng, Y. , … Dong, L. (2020). Immune cell profiling of COVID‐19 patients in the recovery stage by single‐cell sequencing. Cell Discov, 6, 31 10.1038/s41421-020-0168-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werder, R. B. , Lynch, J. P. , Simpson, J. C. , Zhang, V. , Hodge, N. H. , Poh, M. , … Phipps, S. (2018). PGD2/DP2 receptor activation promotes severe viral bronchiolitis by suppressing IFN‐λ production. Science Translational Medicine, 10, eaao0052 10.1126/scitranslmed.aao0052 [DOI] [PubMed] [Google Scholar]
- Whelton, A. (1999). Nephrotoxicity of nonsteroidal anti‐inflammatory drugs: Physiologic foundations and clinical implications. The American Journal of Medicine, 106, 13S–24S. [DOI] [PubMed] [Google Scholar]
- Wilk, A. J. , Rustagi, A. , Zhao, N. Q. , Roque, J. , Martínez‐Colón, G. J. , McKechnie, J. L. , … Blish, C. A. (2020). A single‐cell atlas of the peripheral immune response in patients with severe COVID‐19. Nature Medicine, 26, 1070–1076. 10.1038/s41591-020-0944-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, E. , Walker, A. J. , Bhaskaran, K. J. , Bacon, S. , Bates, C. , Morton, C. E. , … Cockburn, J. (2020). OpenSAFELY: Factors associated with COVID‐19‐related hospital death in the linked electronic health records of 17 million adult NHS patients. MedRxiv. 10.1101/2020.05.06.20092999 [DOI] [Google Scholar]
- Wise, J. (2020). Covid‐19 and thrombosis: What do we know about the risks and treatment? BMJ, 369, m2058. [DOI] [PubMed] [Google Scholar]
- de Wit, E. , Rasmussen, A. L. , Falzarano, D. , Bushmaker, T. , Feldmann, F. , Brining, D. L. , … Munster, V. J. (2013). Middle East respiratory syndrome coronavirus (MERS‐CoV) causes transient lower respiratory tract infection in rhesus macaques. Proceedings of the National Academy of Sciences of the United States of America, 110, 16598–16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Coronavirus disease (COVID‐19). Situation Report–175, July 13, 2020.
- Wu, C. , Chen, X. , Cai, Y. , Xia, J. , Zhou, X. , Xu, S. , … Song, Y. (2020). Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China. JAMA Intern Med., 180, 934 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, D. , & Meydani, S. N. (2008). Age‐associated changes in immune and inflammatory responses: Impact of vitamin E intervention. Journal of Leukocyte Biology, 84, 900–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn, T. A. , & Vannella, K. M. (2016). Macrophages in tissue repair, regeneration, and fibrosis. Immunity, 44, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, Y. , Liu, Y. , Cao, L. , Wang, D. , Guo, M. , Jiang, A. , … Chen, Y. (2020). Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID‐19 patients. Emerg Microbes Infect, 9, 761–770. 10.1080/22221751.2020.1747363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H. , Zhong, L. , Deng, J. , Peng, J. , Dan, H. , Zeng, X. , … Chen, Q. (2020). High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science, 12 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, T. , Gao, X. , Wu, Z. , Selinger, D. W. , & Zhou, Z. (2020). Indomethacin has a potent antiviral activity against SARS CoV‐2 in vitro and canine coronavirus in vivo. BioRxiv. 10.1101/2020.04.01.017624 [DOI] [Google Scholar]
- Yadav, V. , Chi, L. , Zhao, R. , Tourdot, B. E. , Yalavarthi, S. , Jacobs, B. N. , … Kanthi, Y. (2019). Ectonucleotidase tri(di)phosphohydrolase‐1 (ENTPD‐1) disrupts inflammasome/interleukin 1β‐driven venous thrombosis. The Journal of Clinical Investigation, 129, 2872–2877. 10.1172/JCI124804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, T. , Komoto, J. , Watanabe, K. , Ohmiya, Y. , & Takusagawa, F. (2005). Crystal structure and possible catalytic mechanism of microsomal prostaglandin E synthase type 2 (mPGES‐2). Journal of Molecular Biology, 348, 1163–1176. [DOI] [PubMed] [Google Scholar]
- Yan, B. , Chu, H. , Yang, D. , Sze, K.‐H. , Lai, P.‐M. , Yuan, S. , … Yuen, K. Y. (2019). Characterization of the lipidomic profile of human coronavirus‐infected cells: Implications for lipid metabolism remodeling upon coronavirus replication. Viruses, 11, 11 10.3390/v11010073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Q. , Li, P. , Ye, X. , Huang, X. , Mo, X. , Wang, Q. , … Chen, L. (2020). Longitudinal peripheral blood transcriptional analysis of COVID‐19 patients captures disease progression and reveals potential biomarkers. MedRxiv. 10.1101/2020.05.05.20091355 [DOI] [PubMed] [Google Scholar]
- Yan, X. , Hao, Q. , Mu, Y. , Timani, K. A. , Ye, L. , Zhu, Y. , & Wu, J. (2006). Nucleocapsid protein of SARS‐CoV activates the expression of cyclooxygenase‐2 by binding directly to regulatory elements for nuclear factor‐kappa B and CCAAT/enhancer binding protein. The International Journal of Biochemistry & Cell Biology, 38, 1417–1428. 10.1016/j.biocel.2006.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yao, C. , Hirata, T. , Soontrapa, K. , Ma, X. , Takemori, H. , & Narumiya, S. (2013). Prostaglandin E2 promotes Th1 differentiation via synergistic amplification of IL‐12 signalling by cAMP and PI3‐kinase. Nature Communications, 4, 1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, C. , & Narumiya, S. (2019). Prostaglandin‐cytokine crosstalk in chronic inflammation. British Journal of Pharmacology, 176, 337–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, C. , Sakata, D. , Esaki, Y. , Li, Y. , Matsuoka, T. , Kuroiwa, K. , … Narumiya, S. (2009). Prostaglandin E2‐EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nature Medicine, 15, 633–640. 10.1038/nm.1968 [DOI] [PubMed] [Google Scholar]
- Yaqinuddin, A. , & Kashir, J. (2020). Novel therapeutic targets for SARS‐CoV‐2‐induced acute lung injury: Targeting a potential IL‐1β/neutrophil extracellular traps feedback loop. Medical Hypotheses, 143, 109906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasłona, Z. , Pålsson‐McDermott, E. M. , Menon, D. , Haneklaus, M. , Flis, E. , Prendeville, H. , … O'Neill, L. A. J. (2017). The induction of pro‐IL‐1β by lipopolysaccharide requires endogenous prostaglandin E2 production. Journal of Immunology, 198, 3558–3564. 10.4049/jimmunol.1602072 [DOI] [PubMed] [Google Scholar]
- Zeddou, M. , Greimers, R. , de Valensart, N. , Nayjib, B. , Tasken, K. , Boniver, J. , … Rahmouni, S. (2005). Prostaglandin E2 induces the expression of functional inhibitory CD94/NKG2A receptors in human CD8+ T lymphocytes by a cAMP‐dependent protein kinase A type I pathway. Biochemical Pharmacology, 70, 714–724. 10.1016/j.bcp.2005.05.015 [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Guo, R. , Lei, L. , Liu, H. , Wang, Y. , Wang, Y. , … Hu, J. (2020). COVID‐19 infection induces readily detectable morphological and inflammation‐related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome (infectious diseases (except HIV/AIDS)). meRixv. 10.1101/2020.03.24.20042655 [DOI] [Google Scholar]
- Zhang, M.‐Z. , Harris, R. C. , & McKanna, J. A. (1999). Regulation of cyclooxygenase‐2 (COX‐2) in rat renal cortex by adrenal glucocorticoids and mineralocorticoids. PNAS, 96, 15280–15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Tan, Y. , Ling, Y. , Lu, G. , Liu, F. , Yi, Z. , … Chen, J. (2020). Viral and host factors related to the clinical outcome of COVID‐19. Nature, 1–4. [DOI] [PubMed] [Google Scholar]
- Zhao, J. , Zhao, J. , Legge, K. , & Perlman, S. (2011). Age‐related increases in PGD2 expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. The Journal of Clinical Investigation, 121, 4921–4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Zhao, J. , Van Rooijen, N. , & Perlman, S. (2009). Evasion by stealth: Inefficient immune activation underlies poor T cell response and severe disease in SARS‐CoV‐infected mice. PLoS Pathogens, 5, e1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Zhao, Z. , Wang, Y. , Zhou, Y. , Ma, Y. , & Zuo, W. (2020). Single‐cell RNA expression profiling of ACE2, the receptor of SARS‐CoV‐2 (Bioinformatics). bioRixv. 10.1101/2020.01.26.919985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, M. , Gao, Y. , Wang, G. , Song, G. , Liu, S. , Sun, D. , … Tian, Z. (2020). Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cellular & Molecular Immunology, 17, 533–535. 10.1038/s41423-020-0402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, W. , Fan, W. , Zhang, S. , Jiao, P. , Shang, Y. , Cui, L. , … Liu, W. (2019). Naproxen exhibits broad anti‐influenza virus activity in mice by impeding viral nucleoprotein nuclear export. Cell Reports, 27, 1875–1885.e5. 10.1016/j.celrep.2019.04.053 [DOI] [PubMed] [Google Scholar]
- Zhou, F. , Yu, T. , Du, R. , Fan, G. , Liu, Y. , Liu, Z. , … Cao, B. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. Lancet, 395, 1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H. , Chen, X. , Hu, T. , Li, J. , Song, H. , Liu, Y. , … Shi, W. (2020). A novel bat coronavirus closely related to SARS‐CoV‐2 contains natural insertions at the S1/S2 cleavage site of the spike protein. Current Biology, 30, 2196–2203.e3. 10.1016/j.cub.2020.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X.‐L. , Wang, X.‐G. , Hu, B. , Zhang, L. , Zhang, W. , … Shi, Z. L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579, 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, W. , Dowell, D. R. , Huckabee, M. M. , Newcomb, D. C. , Boswell, M. G. , Goleniewska, K. , … Peebles, R. S. (2012). Prostaglandin I2 signaling drives Th17 differentiation and exacerbates experimental autoimmune encephalomyelitis. PLoS ONE, 7, e33518 10.1371/journal.pone.0033518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Fu, B. , Zheng, X. , Wang, D. , Zhao, C. , Qi, Y. , … Wei, H. (2020). Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID‐19 patients. National Science Review, 7, 998–1002. 10.1093/nsr/nwaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z. , Ren, L. , Zhang, L. , Zhong, J. , Xiao, Y. , Jia, Z. , … Wang, J. (2020). Heightened innate immune responses in the respiratory tract of COVID‐19 patients. Cell Host & Microbe, 27, 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla, A. , Chan, J. F. W. , Azhar, E. I. , Hui, D. S. C. , & Yuen, K.‐Y. (2016). Coronaviruses—Drug discovery and therapeutic options. Nature Reviews. Drug Discovery, 15, 327–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, Y. , Zuo, M. , Yalavarthi, S. , Gockman, K. , Madison, J. A. , Shi, H. , … Knight, J. S. (2020). Neutrophil extracellular traps and thrombosis in COVID‐19. JCI Insight, 5, e138999. [DOI] [PMC free article] [PubMed] [Google Scholar]


