Certain respiratory viruses can induce per se immunological changes and morphological alterations that can contribute to the initiation or aggravation of asthmatic processes. New data are emerging on the molecular mechanisms of SARS‐CoV‐2 infection and its possible relation with asthma. In this review, we explore the interrelation between common viruses and asthma, and its potential meaning on the current global pandemic of COVID‐19.
![]()
Keywords: asthma, allergy, COVID‐19, respiratory syncytial virus, rhinovirus, SARS‐CoV‐2
Summary
Viral infections and atopic diseases are closely related and contribute to each other. The physiological deficiencies and immune mechanisms that underlie atopic diseases can result in a suboptimal defense against multiple viruses, and promote a suitable environment for their proliferation and dissemination. Viral infections, on the other hand, can induce per se several immunological mechanisms involved in allergic inflammation capable to promote the initiation or exacerbation of atopic diseases such as atopic asthma. In a world that is affected more and more by factors that significantly impact the prevalence of atopic diseases, coronavirus disease 2019 (COVID‐19) induced by the novel coronavirus severe acute respiratory syndrome (SARS‐CoV‐2) is having an unprecedented impact with still unpredictable consequences. Therefore, it is of crucial importance to revise the available scientific literature regarding the association between common respiratory viruses and asthma, as well as the newly emerging data about the molecular mechanisms of SARS‐CoV‐2 infection and its possible relation with asthma, to better understand the interrelation between common viruses and asthma and its potential meaning on the current global pandemic of COVID‐19.
Abbreviations
- ACE2
angiotensin‐converting enzyme‐2
- AD
atopic dermatitis
- ADEH
AD complicated by eczema herpeticum
- COVID‐19
coronavirus disease 2019
- DCs
dendritic cells
- FcεRI
high‐affinity receptor for IgE
- HBECs
human bronchial epithelial cells
- HDM
house dust mite
- ILC2s
group 2 innate lymphoid cells
- MCs
mast cells
- pDCs
plasmacytoid dendritic cells
- PRRs
pattern recognition receptors
- RSV
respiratory syncytial virus
- RV
human rhinovirus
- SARS‐CoV‐2
severe acute respiratory syndrome
- SOCS1
suppressor of cytokine signalling 1
- Th2
T helper 2
- TLRs
toll‐like receptors
- TMPRSS2
transmembrane protease, serine 2
- TSLP
thymic stromal lymphopoietin
Introduction
Certain atopic diseases such as atopic asthma or atopic dermatitis (AD) have evolved into diseases with higher susceptibility to infections with microorganisms that commonly affect the respiratory system or the skin, respectively. The characteristic CD4 T helper 2 (Th2) polarization that differentiates atopic patients from non‐atopic individuals can impair the ability to mount an effective immune response against viruses. In that respect, Th2 cytokines have been described to be permissive to microbial invasion due to their inhibitory function on epithelial barrier proteins, cell‐mediated immunity, antimicrobial proteins or type I interferon production, which are the first lines of defense against viruses. 1 , 2 In this context, it has been demonstrated that a high proportion of asthmatic patients and individuals with AD complicated by eczema herpeticum (ADEH) have a predisposition to induce a lower production of type I interferons, decreasing in this way the defense against viral infections. 3 , 5 , 6 In line with these observations, an inhibitory effect of Th2 inflammation on the induction of type I interferon, through different mechanisms such as cross‐regulation of the high‐affinity receptor for IgE (FcεRI) and toll‐like receptors (TLRs), inhibition of TLRs by Th2 cytokines, or overexpression of suppressors of cytokine signalling. 7 , 8 Furthermore, the allergic inflammation that characterizes atopic asthma is marked by the infiltration of different immune cells that have the potential to interact with respiratory viruses with an impact on viral infection and asthma exacerbation. 11 , 12
Additionally, data from different studies have revealed that in a background of atopy, IgE‐mediated reactivity against certain respiratory viruses such as respiratory syncytial virus (RSV) and human rhinovirus (RV) might exist. Although the function of IgE against viruses is still not clear, it has been suggested that it may have a role in the exacerbation of asthma induced by respiratory viruses. 13 , 14 In ADEH, IgE against viruses such as herpes simplex virus 1 has also been described. 15 Therefore, there is abundant scientific evidence that indicates that all the aforementioned factors can synergistically contribute to a deficient response to viruses and promote viral dissemination in target organs.
On the other hand, it has been demonstrated that certain viral infections can induce per se the activation of immunological mechanisms and morphological changes such as tissue remodelling that can contribute to the initiation or aggravation of atopic diseases. In line with this assumption, several studies have revealed that epithelial cells infected by specific viruses can induce the production of pro‐Th2 cytokines such as IL‐25 and IL‐33, activating group 2 innate lymphoid cells (ILC2s), dendritic cells (DCs) and Th2 cells, increasing in that way Th2 inflammatory pathways that are linked to allergic inflammation. 16 , 17 In that respect, meta‐analyses demonstrated that severe respiratory viral infections that require hospitalization early in life are associated with an increased risk of asthma development later in life. 18 , 19
In the pandemic of COVID‐19 induced by the novel coronavirus SARS‐CoV‐2, efforts have been made to find potential risk factors. Although the first scientific evidence seems to indicate that allergic asthma is not a strong risk factor for an increased severe infection of SARS‐CoV‐2, several studies have started to explore at a molecular and cellular level the relation between COVID‐19 and asthma. 20 , 21 Yet, little is known regarding the potential consequences and sequelae that SARS‐CoV‐2 infection can produce as such infection is characterized by a strong hyperinflammation and tissue remodelling. For that reason, it is crucial to review the available scientific literature that explores respiratory viruses and their consequences such as the initiation or exacerbation of asthma, as well as the scientific knowledge that is emerging regarding COVID‐19.
In this review, we intend to carry out an in‐depth overview of the current scientific insights about the interconnection between viruses that commonly infect the airways and allergic asthma, and to provide an update of the data regarding the molecular mechanisms of SARS‐CoV‐2 infection and its possible relation with asthma.
Viruses and asthma
The pathophysiology of asthma is characterized by airway inflammation, hyperreactivity and airway remodelling that can lead to a decrease in lung function due to the narrowing of the airways. In the specific case of atopic asthma, there is a predominance of Th2 cells and ILC2s that produce type 2 cytokines involved in allergic inflammation, which is implicated in the initiation and exacerbation of asthmatic processes. 24 However, Th2 immunity alone cannot explain the large pathophysiological events that orchestrate during asthma exacerbation. In that respect, there is growing scientific evidence that viral infections of the respiratory tract, especially the ones produced by single‐stranded RNA viruses, are associated with asthma, particularly in the paediatric population, although also in adult patients. 25 The association between respiratory viruses and asthma has been analysed during the years from two different perspectives. On one hand, a branch of research has focused on the impact that respiratory viral infections have on the development and/or exacerbation of asthma. On the other hand, a wide spectrum of scientific research has focused on the study of asthma as a risk factor for the development of repeated and severe respiratory viral infections due to the physiological deficiencies and immune mechanisms that underlie the disease.
The clinical association between respiratory viral infections and asthma
Respiratory syncytial virus and RV belonging to Paramyxoviradae and Picornaviradae family, respectively, are single‐stranded RNA viruses involved in the infectious processes of the human respiratory tract. They are the respiratory viruses that have been more commonly associated with the development of asthma or asthma exacerbation. Other viruses such as influenza virus also seem to have an impact on asthma, while coronavirus, adenovirus, parainfluenza virus, metapneumovirus or bocaviruses have been described as potential risk factors for asthma exacerbations, although at a lower extend. 26 , 27
The first scientific evidence that associated respiratory viral infections and asthma development or exacerbation came from human epidemiological studies in the early 1970s, especially carried out in paediatric populations. Subsequent experimental studies using human samples and animal models started to explore the mechanisms involved in such association. Data from epidemiological studies have shown that most children under the age of 2 years have been infected at least once with RSV, which is a highly contagious virus that produces infections that in most cases cause mild symptomatology. However, about 2−3% of the patients infected with RSV have severe symptoms for which hospitalization is needed. 28 Early studies in the 1970s and 1980s started to suggest an association between RSV infection and the development of asthma. 29 , 30 In the following years, several other studies supported this hypothesis. In this context, a meta‐analysis carried out in 2013 examined 15 studies performed from 1978 to 2012 that included 82 008 paediatric individuals in whom the association between hospitalization due to infection of the lower respiratory tract produced by RSV in the first 3 years of life and the risk of asthma development was studied. The meta‐analysis showed that 21·9% of the paediatric patients hospitalized for RSV infection developed asthma as a sequela during the first 5 years of life. It was also estimated that from the total of asthmatic children under the age of 5 years, the ones that had been infected by RSV requiring hospitalization represent 6·4%. Although the meta‐analysis showed a strong association between RSV hospitalization in the first years of life and the development of asthma, follow‐up studies demonstrated that such a connection was reduced by age over time. 19 However, this observation was challenged by further systemic reviews that found a continued substantial risk of asthma in adulthood in those patients hospitalized for RSV infection early in life. Such risk was in part due to a late onset of asthma that may not be analysed in all the follow‐up studies. Differences in the definition of asthma among the studies could also be responsible for such discrepancies. 31
Human rhinovirus has also been identified as one of the respiratory viruses mainly associated with acute asthma development and/or exacerbations. Based on its genetics, RV can be classified in species RV‐A, ‐B and ‐C, which are composed of more than 160 types. Typically, RV is associated with infection of the upper respiratory tract, although scientific evidence has shown that the lower tract can also be a target for RV. The connection between RV infection and the development of asthma has been analysed in several epidemiological studies. A meta‐analysis carried out in 2017 included 15 studies that involved paediatric patients under the age of 3 years that were diagnosed with wheezing associated with RV infection and had a follow‐up study to evaluate the development of asthma. The meta‐analysis revealed that there was an association between RV wheezing diagnosed in the first 3 years of life and an increased risk of development of asthma later in life. Interestingly, such association was still significant with increased age (≥ 10 years). 18
The clinical association between respiratory viral infections and asthma can also be analysed from the perspective of the predisposition that an asthmatic background has for the development of a higher number and/or more severe viral infections in the airways. In that respect, data from several clinical studies have revealed that atopic paediatric patients may have a higher risk of adverse responses to respiratory viral infections, such as the ones produced by RV, and those infections are significantly related with wheezing in children that were hospitalized due to asthma. 32 , 33 In young adults it has also been found that an asthmatic background with high levels of total IgE seems to be predisposed to exacerbated responses to acute infections with viruses such as RV. 34
Besides RV and RSV, other respiratory viruses such as influenza virus, coronaviruses, parainfluenza virus, adenovirus or metapneumovirus might be associated with asthma initiation or exacerbation. 26 , 35 However, although previous studies have found that certain strains of coronaviruses can be associated with asthma exacerbation, this link seems to be weaker than for other respiratory viruses such as RV or RSV. 36 , 37
In the case of COVID‐19 induced by the novel coronavirus SARS‐CoV‐2, a single‐stranded RNA virus, the symptoms can range from fever, cough and shortness of breath to severe pneumonia that can compromise lung function requiring mechanical ventilation. 38 Although the true mortality rate of COVID‐19 is still currently difficult to estimate, the first data given by the World Health Organization considering the crude mortality ratio (reported deaths divided by reported cases) situated the figure between 3% and 6%. 39 Risk factors such as advanced age, hypertension, respiratory disease, cardiovascular disease, diabetes, male sex or high levels of lactate dehydrogenase have been associated with severe COVID‐19. 40 , 41 Obesity has also been pointed out as a risk factor. 42 , 43 The first scientific evidence seems to indicate that asthma does not seem to constitute a risk factor for an enhanced infection or aggravation of COVID‐19. 44 Two studies that have analysed the prevalence of asthma in two cohorts of COVID‐19 patients in Wuhan, China have established such prevalence in 0−0·9%. 41 , 45 These results were further confirmed in study populations from other countries. 46 However, recent data from a large cohort of patients in the USA demonstrated that asthma was significantly linked to a longer duration of intubation in patients with COVID‐19 younger than 65 years. 47 In a prospective study carried out in the UK, it was found that the prevalence of asthma in 605 patients with COVID‐19 was 17·9%, which is greater than the prevalence found in the general population. 48 These data suggest that asthma may be a greater risk factor for COVID‐19 than previously suggested. 49 , 50 In this context, different research groups have begun to analyse at a molecular and cellular level the relation of asthma and COVID‐19. Very recent studies have shown that Th2 cytokines have a role in the modulation of the entry receptor of SARS‐CoV‐2, angiotensin‐converting enzyme‐2 (ACE2) in the airways and transmembrane protease, serine 2 (TMPRSS2), the host protease that facilitates the binding of SARS‐CoV‐2 to its receptor. Studies found that the expression of TMPRSS2 in the airway epithelium was greatly upregulated by Th2 cytokines, especially by IL‐13; however, the same Th2 cytokines decreased the levels of ACE2 in nasal and bronchial epithelium. Respiratory allergy was also linked to a significant decrease in the expression of ACE2. 20 , 21 Interestingly, in a large cohort of asthmatic patients in the USA, it was found that the expression of ACE2 and TMPRSS2 was specifically increased in sputum cells of males, African Americans individuals, and patients suffering from diabetes mellitus. 51 From these results, it seems that Th2 cytokines have differential effects on two key proteins involved in the infective mechanisms of SARS‐CoV‐2. Moreover, an asthmatic background seems to modulate these two proteins differently depending on the group of patients in question (male versus female or African Americans versus Caucasians). However, and attending to the so‐far reported cases of asthma among patients with COVID‐19, the globally available data seem to indicate that Th2 inflammation linked to atopic asthma may not have a strong implication in a severe course of the disease. 20 , 21
Despite these facts, SARS‐CoV‐2 is a new coronavirus that infects humans, and little information is available regarding the possible outcomes derived from SARS‐CoV‐2 infection in humans and the potential effects on the initiation or exacerbation of diseases such as asthma. It is known that the most severe form of COVID‐19 seems to be linked to a ‘cytokine storm’ leading to a hyperinflammation that can induce severe injuries in the lungs in a way that the disease, in the cases that overcome it, can produce important sequelae in multiple organs or tracts, such as the respiratory tract. 52 , 53
In the following sections, we will focus on the available scientific knowledge regarding the molecular mechanisms that underlie the association between respiratory viruses that normally infect humans, such as RV, RSV, influenza virus and asthma. These findings could give important insights and directions about the potential impact that SARS‐CoV‐2 can have on patients who are currently suffering from this infection. In the bidirectional and probably interconnected association between respiratory viruses, such as RV, RSV, influenza virus and asthma, multiple molecular and cellular mechanisms have been described that could explain the interrelation between them. These mechanisms will be described in the following sections, differentiating, on the one hand, the mechanisms involved in the impact that viral infections can potentially have on the initiation or exacerbation of asthma (respiratory viral infections as a risk factor for asthma development: mechanisms involved), and on the other hand, the mechanisms that could explain why an asthmatic phenotype seems to be more likely to suffer from infections produced by respiratory viruses (asthma as a risk factor for respiratory viral infections: mechanisms involved).
Respiratory viral infections as a risk factor for asthma development: mechanisms involved
Different mechanisms have been suggested to be involved in the initiation or exacerbation of asthma as a consequence of respiratory viral infections. Although the severity and the outcomes of a respiratory viral infection depend on factors such as the host immune system or environmental aspects, it has been observed that respiratory viral infections can induce per se immunological changes and morphological alterations that can contribute to the initiation or aggravation of asthmatic processes (Fig. 1). Infections produced by respiratory viruses such as RSV or RV are characterized by airways inflammation and tissue remodelling that could have an impact on the initiation of asthmatic processes.
Figure 1.
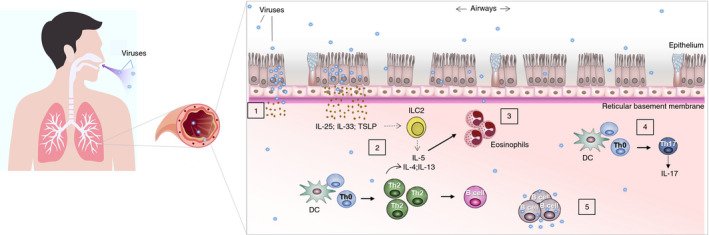
Mechanisms involved in the infection of respiratory viruses that can promote asthma development. Respiratory viruses can induce immunological and morphological changes that can contribute to the development of asthmatic processes. (1) Respiratory viruses replicate in the airway epithelium, which can alter the epithelial barrier integrity. (2 and 3) Infected epithelial cells promote the production of pro‐inflammatory cytokines such as IL‐25, IL‐33 and thymic stromal lymphopoietin (TSLP), which induce the activation of group 2 innate lymphoid cells (ILC2s), dendritic cells (DCs), T helper 2 (Th2) cells, increasing Th2 inflammation linked to atopic asthma. (4) During respiratory viral infections, the cytokine IL‐17, a pro‐inflammatory cytokine produced by Th17 cells and linked to asthma, can increase. (5) Respiratory viruses can infect immune cells such as B‐cells, macrophages or T‐cells, which may contribute to viral replication and propagation.
Induction of pro‐inflammatory cytokines derived from the airway’s epithelium during viral infection
The epithelial barrier in the airways is the first place for viral replication and propagation, which can seriously alter its integrity and homeostasis, and derive in the release of pro‐inflammatory cytokines and increased allergen sensitization. In that respect, several studies have revealed that epithelial cells infected by respiratory viruses can promote the production of cytokines such as IL‐25, IL‐33 and thymic stromal lymphopoietin (TSLP) activating ILC2s, DCs and Th2 cells, increasing Th2 inflammatory pathways linked to allergic inflammation (Fig. 1). 16 , 17 , 54 Support for this assumption comes from observations gained in mouse models in which cytokines derived from the airway epithelium such as IL‐25, IL‐33 and TSLP were produced rapidly upon viral infections with respiratory viruses such as RV or RSV. The epithelial‐derived cytokines production went along with an augmented number of ILC2s, which are important producers of IL‐13, deriving in allergic inflammation. The blockade of IL‐25, IL‐33, TSLP or their receptors in neonatal mice exposed to respiratory viruses decreased Th2 allergic inflammation, hyperresponsiveness of the airways, eosinophilia and mucus production. 55 , 56 In that sense, the induction of ILC2s through IL‐25, IL‐33 and TSLP during RSV and RV infection has been found to be key for RSV and RV‐induced asthma. IL‐13‐producing ILC2s during respiratory viral infections are an indicator of the severity of the disease. 54 , 55 Additional cytokines and compounds with regulatory effect over ILC2s activation have been described during cell injury associated with respiratory viral infections. In that respect, during RSV infection, an increase in the production of IL‐1β, a regulator of ILC2s, has been observed with a potential role in the initiation of asthma. 58 Uric acid is also produced during cell injury associated with RSV infection. This compound induced the production of reactive oxygen species and the activation of the NLRP3 inflammasome, with an effect on IL‐1β production and consequent ILC2s activation. Uric acid has been associated with pulmonary injury and asthma. Inhibition of uric acid or IL‐1β in murine models of RSV infection reduced the production of mucus, infiltration of ILC2s, type 2 immune response, and development of asthma during RSV infection and allergen challenges, which highlight their importance in virus‐induced asthma. 59
Role of other inflammatory pathways in virus‐induced asthma
In the initiation of asthma, other immune mechanisms besides or in a combination with type 2 inflammation can take place, such as neutrophilic inflammation in which Th1 and Th17 cells play an important role. In this context, it has been observed that asthma can be initiated by respiratory viruses by the activation of immune mechanisms different from the ones involved in type 2 inflammation, although they can also act in combination with this type of inflammation. In that respect, in a mouse model of virus‐induced asthma, the signalling of IL‐1β has been implicated in neutrophilic inflammation. 60 IL‐17, a pro‐inflammatory cytokine produced by Th17 cells, which is known to contribute to asthma by the induction of lung inflammation, mucus production and promotion of Th2 responses, has been found to be augmented during RSV infections and to play a key role in the pathogenesis of asthma induced by RSV. 61 Other studies have confirmed that Th17 and Th2 pathways seem to be implicated in the response to RSV infection, which can derive in persistent type 2 immunity implicated in asthma. 61 , 62
Replication of respiratory viruses in immune cells with an impact on asthma
Respiratory viruses can also have an impact on asthma development due to their interaction with different immune cells. Epithelial cells infected by respiratory viruses such as RV produce inflammatory factors that promote chemotaxis, proliferation and activation of specific immune cells, amplifying in that way inflammatory processes. Although it was previously suggested that RV is not able to infect immune cells, recent studies have argued against this concept, and have demonstrated that RV can infect macrophages, 65 T‐cells 66 , 67 or mast cells (MCs), which can act as viral reservoirs and release viral particles contributing in that way to viral infection. 11 Due to the proximity of infiltrated inflammatory immune cells with the epithelium from the upper and lower airways, it is reasonable to expect that those cells also have a role in RV infection with a potential impact on asthma initiation or exacerbation. In that respect, a study showed that RV interacted with CD19 + B‐cells, CD4 + T‐cells, CD8 + t‐cells and monocytes in vitro. RV was able to be internalized by CD19 + B‐cells, to induce their proliferation, and to form viral replication centres in which the newly released virions were able to infect other cells (Fig. 1). Using peripheral blood mononuclear cells (PBMCs), it was found that RV induced their activation and production of inflammatory cytokines, such as IL‐6, TNF‐α, IFN‐α, IFN‐γ, IL‐27, MIP‐1β, IL‐1β and RANTES. 68
Influence of viral infection on airway microbiota and epithelial barrier integrity
The compositions of the microbiota in the upper and lower respiratory tracts (URT and LRT, respectively) have been found to show similarities; however, they differ in a way that the density of microorganisms tends to be higher in the URT than in the LRT in healthy individuals. Data from different studies have shown that asthmatic patients have an increase in the population of different microbial communities in the lungs with an enrichment in the abundance of specific species. 69 , 70 Interestingly, infections by respiratory viruses also have a relevant impact on the airway microbiota. In that respect, it has been found that respiratory viruses can modify the composition of the airway microbiota in a sense that can promote the growth of pathogens that can affect not only the URT but also the LRT with a potential impact on asthma exacerbation. In this context, it has been demonstrated that certain respiratory viruses can increase the adherence of pathogenic bacteria to the lung epithelium with a promotion of their entrance into the LRT leading to potential secondary bacterial infections. 71 , 72 The infections produced by respiratory viruses can also alter the innate immunity against bacteria, impairing its clearance. 73 The relation of respiratory viruses and airway microbiota has been shown to be reciprocal, and ample scientific evidence has demonstrated that airway microbiota can also alter the course of viral infections through the influence that it exerts on the host immune responses. In that respect, data from animal models have shown that commensal microbiome in the URT has a protective function against the airway hyperresponsiveness induced by RSV, and the use of antibiotics that decreases specific populations in the URT microbiome reverts this effect. 74 The same effect has also been found for other respiratory viruses. 75
Respiratory viruses can also compromise the structural integrity of the epithelial barrier during their infective processes. In that respect, the replication of certain respiratory viruses in epithelial cells can induce loss of ciliated cells, which derives in morphological damages that can promote airway hyperresponsiveness and aggravation of asthmatic processes. 76 , 77 Apoptotic mechanisms in the epithelial barrier induced in the host, which initially has the function of limiting viral spreading, can cause further damage to the structure of the airway epithelium. Scientific evidence has also shown that respiratory viruses can disrupt tight junctions in the epithelium, disturbing cell‐to‐cell connections with an increase in paracellular permeability. 78 , 79 Furthermore, certain respiratory viruses also have the capacity to interfere with processes of repair and wound healing in the airway epithelium, which can further alter the integrity and homeostasis of the epithelial barrier. 80
Asthma as a risk factor for respiratory viral infections: mechanisms involved
As has been already mentioned, asthma pathophysiology is complex, and several of the mechanisms that underlie the disease have been pointed out as facilitators of the spreading and infection of respiratory viruses with an impact on asthma exacerbations. In that context, the predominant Th2 inflammation, which is a hallmark of atopic asthma and is characterized by Th2 cytokines IL‐4, IL‐5 and IL‐13 produced by Th2 cells and ILC2s, has been associated with impaired protection against viruses. Th2 inflammation constitutes an immune response that is not involved in the immune defense against viruses. Not only is it a response that is not involved in virus protection but, also, Th2 inflammation can promote the inhibition of different mechanisms involved in the protection against viruses (Fig. 2). 81 Furthermore, the characteristic airway inflammation, bronchial obstruction and hyperresponsiveness that underlie asthmatic processes represent a suitable environment for the widespread of specific respiratory viruses.
Figure 2.
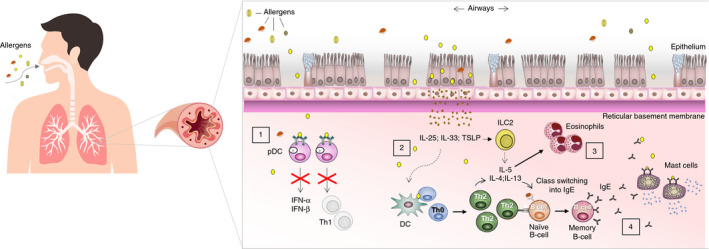
Pathophysiological mechanisms of asthma that may act as facilitators of respiratory viral infections. (1) An impaired production of type I interferons (IFN‐α/IFN‐β) has been found in a high proportion of asthmatic patients. One of the mechanisms involves crosslinking of IgE bound to the high‐affinity receptor of IgE (FcεRI) by allergens in plasmacytoid dendritic cells (pDCs) that result in decreased toll‐like receptor (TLR) expression and a decline in type I interferons production. (2) T helper 2 (Th2) inflammation in asthma linked to the production of IL‐4, IL‐5 and IL‐13 by Th2 cells and group 2 innate lymphoid cells (ILC2s) has been related to reduced protection against viruses. Furthermore, allergen exposure can induce production in the epithelium of cytokines that can synergistically interact with respiratory viruses. (3) Asthma is characterized by abundant infiltration of immune cells that can act as infective targets for respiratory viruses. (4) In the context of Th2 polarization in atopic asthma, high amounts of specific IgE against different allergens are produced. Recent studies have proposed that specific IgE against certain viruses can also be produced.
Decrease of type I interferons in asthma
Type I interferons (IFN‐α/IFN‐β) are antiviral cytokines that constitute the first line of defense against viruses. They are induced upon ligation of viral components to pattern recognition receptors (PRRs) expressed in different cell types. An extensive body of scientific evidence has demonstrated that a high proportion of asthmatic patients has a predisposition to produce lower levels of type I interferons upon respiratory viral infections, decreasing in that way the defense against viral infections. 5 , 6 Such impairment has been suggested to depend on the specific site of the respiratory tract, with higher impact on cells from the lower airways, and on the different stages of asthma or its phenotypes, with opposite effect (overproduction of type I interferons) in specific asthmatic phases or phenotypes. 82 , 83 The impairment may also depend on the specific respiratory virus that infects the airways. 83 However, attending to the findings of numerous studies, it seems to be clear that in at least a subgroup of patients with asthma, an impairment of type I interferon production exists with a specific role in increased viral load, infection severity and perpetuation of Th2 inflammation due to lack of the counter‐regulation effect of type I interferons. 81 , 84 , 85 Considerable research has been performed to elucidate the mechanisms involved in the deficient production of interferons in a subgroup of asthmatic patients. The association of allergen exposure and a lower expression of type I interferons has been suggested. In this context, a cross‐regulation mechanism between FcεRI and TLRs in certain cell types such as plasmacytoid dendritic cells (pDCs) has been described, which may explain why the crosslinking of IgE bound to FcεRI by allergens may result in a reduced TLR expression and ultimately in a decreased capacity to secrete type I interferons for viral defense (Fig. 2). 7 Furthermore, the concept of a deficient production of type I interferons in asthmatic patients associated with an inhibitory effect of the IgE crosslinking in pDCs has been further analysed. A recent study found that PBMCs and pDCs from paediatric patients with exacerbated asthma increased the production of IFN‐α in the presence of Omalizumab (an anti‐IgE antibody) when they were cultured with RV, influenza virus and with IgE crosslinking. Omalizumab also decreased the expression of FcεR1α on pDCs, and such a decrease was linked with a lower exacerbation of asthma. 86 Using human bronchial epithelial cells (HBECs) and a mouse model of asthma exacerbation, it was found that the exposure to house dust mite (HDM) before viral infection led to a decreased interferon response in HBECs and the mouse model of asthma. Such an impaired response was mediated by the direct effect of HDM on the signalling pathway of TLR3, which suggests that allergens can impact on PRRs and their normal antiviral function. 87 Other components involved in Th2 inflammation such as the Th2 cytokines IL‐4 and IL‐5 have also been implicated in the inhibition of TLR3 expression and its signalling through interferon‐responsive factor 3, which decreased type I interferons in response to RV. 8 All these findings suggest that Th2 inflammation characteristic for allergic asthma has an inhibitory effect on the induction of type I interferons production. Another interesting connection between asthma and a lower type I interferon production arises from studies that analysed the expression of suppressor of cytokine signalling 1 (SOCS1), a regulator that negatively controls type I and type II interferon production. The expression of SOCS1 can be induced by Th2 cytokines and specific respiratory viruses. SOCS1 was found to be increased in the epithelium from the bronchi of asthmatics, and its expression seemed to be linked to asthma severity. In HBECs obtained from asthmatic individuals and infected with RV, an increased expression of SOCS1 accompanied by interferon deficiency and enhanced RV replication were found. 9 , 10 SOCS1 had a direct role in the suppression of interferons production upon RV infection in a mouse model. 9
Pro‐Th2 epithelial‐derived cytokines in asthma synergistically interact with respiratory viruses
The exposure to allergens in the airways of asthmatic patients induces alterations in the epithelium that promote an increase of pro‐Th2 epithelial‐derived cytokines. Data from different studies have demonstrated that these epithelial‐derived cytokines in asthma synergistically interact with respiratory viruses to perpetuate allergic inflammation and to promote asthma exacerbations (Fig. 2). 88 That is the case of the epithelial cytokine IL‐33, which is involved in the initiation of allergic inflammation upon allergen encounter in atopic individuals. Recently it has been demonstrated that the major local sources of IL‐33 during asthma exacerbations in the presence of influenza virus were bronchial ciliated cells in patients with mild asthma. The study found that IL‐33 in contrast to TSLP suppressed the expression of IFN‐β in DCs and epithelial cells, having IL‐33 inhibitory action on innate as well as on adaptive immunity against influenza virus, which increased viral load and viral spread. Consequently, IL‐33 produced increased hyperresponsiveness of the airways and allergic inflammation. 89 Similar results were found using a Pneumovirus in a mouse model of asthma induced by cockroach extract. 90 In the case of RV, it has been observed that IL‐33 together with IL‐4, IL‐5 and IL‐13 were induced in the airways of asthmatic patients during RV infection, but not in healthy individuals that were also exposed to RV. In asthmatic patients, there was a correlation between IL‐33 levels and IL‐5, IL‐13 and viral load. 16 In line with this observation, it has been found that IL‐33 exposure to human circulating leucocytes incubated with RV increased IL‐5 and IL‐13 production by leucocytes from asthmatic patients but not from healthy individuals. IL‐33 also increased ST2 surface expression, which is the IL‐33 receptor, in asthmatic patients but not in healthy subjects, being ST2 + innate lymphoid cells the cell types involved in the increased release of IL‐13. In that way, IL‐33 and respiratory viruses such as RV seem to interact in a synergistically way in asthmatic patients with a potential contribution to asthma exacerbations. 91 Interestingly, the chronic expression of IL‐33 in epithelial cells from asthmatic patients was associated with a decreased antiviral response against RV together with an increased RV‐induced exacerbation. The study also found that the blockade of IL‐33 reduced type 2 inflammation, viral load, and promoted antiviral responses in a preclinical mouse model of chronic asthma and RV infection. The use of anti‐IL‐33 antibody also produced a decrease in RV replication and increased antiviral cytokines in human airway epithelial cells from asthmatic patients. 92
Immune cells in asthma with a role in viral infections
Allergic inflammation that underlies atopic asthma is characterized by the infiltration of different immune cells that can interact with respiratory viruses with a potential impact on viral infection and/or asthma exacerbation (Fig. 2). In that respect, it is known that MC numbers are increased in asthmatic patients. MCs can also be found in the bronchial epithelium of asthmatics during experimental RV infection. 93 Data from a study that utilized human MC line LAD2 and primary human cord blood‐derived MCs revealed that MCs mounted an innate response upon RV infection; however, and despite this fact, MCs were permissive for the replication of RV and they were able to release viral particles, acting as a reservoir for RV that can potentially contribute to asthma exacerbation induced by RV. 11 Eosinophils are another cell type that have a pro‐inflammatory role in asthmatic processes as they produce cytotoxic compounds and reactive oxygen species that contribute to tissue damage. It has been observed that during viral infections in asthmatic patients, eosinophils tend to accumulate in the airways, which is also a hallmark of atopic and non‐atopic asthma. A recent study found that RSV and influenza viruses can adhere to human eosinophils and are inactivated after their internalization by eosinophils. Interestingly, eosinophils from asthmatic patients showed an impaired capacity to interact and capture viral particles compared with eosinophils from healthy controls, and this reduced capacity correlated with asthmatic severity. The reduced antiviral function of eosinophils in asthmatic patients during respiratory viral infections could contribute to virus‐induced asthma exacerbations. 12 In line with this observation, it has been shown that the frequency of M1‐like macrophages, which are involved in antiviral responses through type I and III interferon production, were decreased in asthmatic patients during asthma exacerbation induced by RV. 94 These results seem to indicate that several immune cells that normally have an antiviral function in healthy individuals are found in lower frequency in asthmatic patients or with an altered functionality, thus promoting respiratory viral infection with detrimental consequences for asthmatic symptoms.
Specific IgE against viruses in asthma
Another mechanism that connects atopy and viral infection is based on the fact that in a background of atopy, specific IgE against certain viruses can be produced and, although its function is not exactly known, it has been suggested that antiviral IgE could have a possible link with the exacerbation of allergic asthma. 95 , 96 The phenotype of allergic asthma, characterized by a greater Th2 polarization and relative Th1 deficiency, may promote the production of IgE against respiratory viruses (Fig. 2). In that respect, studies in mice have demonstrated that specific IgE against respiratory viruses such as RSV can be produced, and they have an important role in the increase of Th2 inflammation in the lungs and exaggerated responsiveness in the airways. 97 , 98 That antiviral IgE may constitute a maladaptive immune response against respiratory viruses that would circumvent classical viral immune‐protective responses with a possible promotion of disseminated viral infection in the airways. In this context, it has been demonstrated in human studies that IgE against RSV is made as part of the antiviral response to RSV infection, and the levels of IgE anti‐RSV were found to be higher in asthmatic subjects compared with non‐asthmatic individuals. 13 Another study found IgE against RV in the sera of subjects who have been exposed to them. 14 Therefore, there is growing evidence that antiviral IgE produced as part of an antiviral immune response may contribute to the exacerbation or even development of atopic diseases, although further studies in human samples should be carried out to support this hypothesis. 14
Conclusions
Many of the mechanisms that underlie asthma not only have a detrimental effect on the airways of asthmatic patients, but can also decrease or block an adequate immune response to viral respiratory infections. This effect might lead to an aggravation of the asthmatic status. This is the case in infections caused by RSV, RV or influenza virus. Interestingly, the first scientific evidence indicates that an asthmatic phenotype does not appear to be a strong risk factor for an increased severe infection with the new coronavirus SARS‐CoV‐2. However, little is known about the effects and sequelae that the infection caused by SARS‐CoV‐2 can produce in the lung function of patients who have overcome the disease. In this review, it has been detailed that certain respiratory viral infections can induce per se immunological changes and morphological alterations that can contribute to the initiation or aggravation of asthmatic processes. For that reason, during the pandemic caused by COVID‐19, which is impacting the world in a way rarely seen before and is characterized by lung damage, ‘cytokine storm’ and hyperinflammation, it is of vital importance to monitor and exhaustively evaluate the consequences and sequelae that COVID‐19 can produce in the patients who went through the disease.
Disclosure
The authors declare no competing interests.
Acknowledgements
B.C. is funded in the senior modality of the research program Talento Investigador of the Community of Madrid (Regional Ministry of Science, Universities, and Innovation, Madrid, Spain; grant number: 2019‐T1/BIO‐12690) and by a grant from strategic health action (AES), Carlos III Health Institute (Spanish Ministry of Science and Innovation): grant number: PI20/00351.
Data Availability Statement
No data are available.
References
- 1. Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387:1109–22. [DOI] [PubMed] [Google Scholar]
- 2. Cabanillas B, Novak N. Atopic dermatitis and filaggrin. Curr Opin Immunol 2016; 42:1–8. [DOI] [PubMed] [Google Scholar]
- 3. Peng WM, Jenneck C, Bussmann C, Bogdanow M, Hart J, Leung DY, et al Risk factors of atopic dermatitis patients for eczema herpeticum. J Invest Dermatol 2007; 127:1261–3. [DOI] [PubMed] [Google Scholar]
- 4. Hinz T, Zaccaro D, Byron M, Brendes K, Krieg T, Novak N, et al Atopic dermo‐respiratory syndrome is a correlate of eczema herpeticum. Allergy 2011; 66:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, et al Innate immune responses to rhinovirus are reduced by the high‐affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol 2012; 130:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza‐Stanca V, et al Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005; 201:937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, et al Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol 2010; 184:5999–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Contoli M, Ito K, Padovani A, Poletti D, Marku B, Edwards MR, et al Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy 2015; 70:910–20. [DOI] [PubMed] [Google Scholar]
- 9. Gielen V, Sykes A, Zhu J, Chan B, Macintyre J, Regamey N, et al Increased nuclear suppressor of cytokine signaling 1 in asthmatic bronchial epithelium suppresses rhinovirus induction of innate interferons. J Allergy Clin Immunol 2015; 136:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bedke N, Sammut D, Green B, Kehagia V, Dennison P, Jenkins G, et al Transforming growth factor‐beta promotes rhinovirus replication in bronchial epithelial cells by suppressing the innate immune response. PLoS One 2012; 7:e44580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akoto C, Davies DE, Swindle EJ. Mast cells are permissive for rhinovirus replication: potential implications for asthma exacerbations. Clin Exp Allergy 2017; 47:351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sabogal Piñeros YS, Bal SM, Dijkhuis A, Majoor CJ, Dierdorp BS, Dekker T, et al Eosinophils capture viruses, a capacity that is defective in asthma. Allergy 2019; 74:1898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith‐Norowitz TA, Mandal M, Joks R, Norowitz LT, Weaver D, Durkin HG, et al IgE anti‐respiratory syncytial virus antibodies detected in serum of pediatric patients with asthma. Hum Immunol 2015; 76:519–24. [DOI] [PubMed] [Google Scholar]
- 14. Tam JS, Jackson WT, Hunter D, Proud D, Grayson MH. Rhinovirus specific IgE can be detected in human sera. J Allergy Clin Immunol 2013; 132:1241–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cabanillas B, Weighardt H, Izquierdo E, Förster I, Novak N. IgE reactivity against herpes simplex virus 1 in patients with atopic dermatitis complicated by eczema herpeticum. Allergy 2020; 75:226–9. [DOI] [PubMed] [Google Scholar]
- 16. Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo‐Torralbo MB, Footitt J, et al IL‐33‐dependent type 2 inflammation during rhinovirus‐induced asthma exacerbations in vivo. Am J Respir Crit Care Med 2014; 190:1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beale J, Jayaraman A, Jackson DJ, Macintyre JDR, Edwards MR, Walton RP, et al Rhinovirus‐induced IL‐25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med 2014; 6:256ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu L, Pan Y, Zhu Y, Song Y, Su X, Yang L, et al Association between rhinovirus wheezing illness and the development of childhood asthma: a meta‐analysis. BMJ Open 2017; 7:e013034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Regnier SA, Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta‐analysis. Pediatr Infect Dis 2013; 32:820–6. [DOI] [PubMed] [Google Scholar]
- 20. Sajuthi SP, DeFord P, Jackson N, Montgomery M, Everman J, Rios C, et al Type 2 and interferon inflammation strongly regulate SARS‐CoV‐2 related gene expression in the airway epithelium. bioRxiv 2020; 10.1101/2020.04.09.034454. [DOI] [PMC free article] [PubMed]
- 21. Jackson DJ, Busse WW, Bacharier LB, Kattan M, O'Connor GT, Wood RA, et al Association of Respiratory Allergy, Asthma and Expression of the SARS‐CoV‐2 Receptor, ACE2. J Allergy Clin Immunol 2020; 146:203–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kimura H, Francisco D, Conway M, et al Type 2 Inflammation Modulates ACE2 and TMPRSS2 in Airway Epithelial Cells. J Allergy Clin Immunol 2020; 146:80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bradding P, Richardson M, Hinks TSC, Howarth PH, Choy DF, Arron JR, et al ACE2, TMPRSS2 and furin gene expression in the airways of people with asthma – implications for COVID‐19. J Allergy Clin Immunol 2020; 146:208–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet 2018; 391:783–800. [DOI] [PubMed] [Google Scholar]
- 25. Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol 2017; 140:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khetsuriani N, Kazerouni NN, Erdman DD, Lu X, Redd SC, Anderson LJ, et al Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol 2007; 119:314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arden KE, Faux CE, O'Neill NT, McErlean P, Nitsche A, Lambert SB, et al Molecular characterization and distinguishing features of a novel human rhinovirus (HRV) C, HRVC‐QCE, detected in children with fever, cough and wheeze during 2003. J Clin Virol 2010; 47:219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr 2003; 143:S112–117. [DOI] [PubMed] [Google Scholar]
- 29. Welliver RC, Kaul A, Ogra PL. Cell‐mediated immune response to respiratory syncytial virus infection: relationship to the development of reactive airway disease. J Pediatr 1979; 94:370–5. [DOI] [PubMed] [Google Scholar]
- 30. Pullan CR, Hey EN. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br Med J (Clin Res Ed) 1982; 284:1665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Szabo SM, Levy AR, Gooch KL, Bradt P, Wijaya H, Mitchell I. Elevated risk of asthma after hospitalization for respiratory syncytial virus infection in infancy. Paediatr Respir Rev 2013; 13(Suppl 2):S9–15. [DOI] [PubMed] [Google Scholar]
- 32. Heymann PW, Carper HT, Murphy DD, Platts‐Mills TA, Patrie J, McLaughlin AP, et al Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol 2004; 114:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rawlinson WD, Waliuzzaman Z, Carter IW, Belessis YC, Gilbert KM, Morton JR. Asthma exacerbations in children associated with rhinovirus but not human metapneumovirus infection. J Infect Dis 2003; 187:1314–8. [DOI] [PubMed] [Google Scholar]
- 34. Zambrano JC, Carper HT, Rakes GP, Patrie J, Murphy DD, Platts‐Mills TA, et al Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol 2003; 111:1008–16. [DOI] [PubMed] [Google Scholar]
- 35. Kurai D, Saraya T, Ishii H, Takizawa H. Virus‐induced exacerbations in asthma and COPD. Front Microbiol 2013; 4:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greenberg SB. Update on Human Rhinovirus and Coronavirus Infections. Semin Respir Crit Care Med 2016; 37:555–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zheng XY, Xu YJ, Guan WJ, Lin LF. Regional, age and respiratory‐secretion‐specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review. Arch Virol 2018; 163:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al Team CNCIaR. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. World Health Organization . Coronavirus disease 2019 (COVID‐19) Situation Report – 46, 2020. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports. Accessed 23 May 2020.
- 40. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta‐analysis. Int J Infect Dis 2020; 94:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol 2020; 146:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang R, Zhu L, Xue L, Liu L, Yan X, Wang J, et al Clinical Findings of Patients with Coronavirus Disease 2019 in Jiangsu Province, China: A Retrospective, Multi‐Center Study. SSRN Electronic J 2020; 14:e0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al Novel Coronavirus‐Infected Pneumonia in Wuhan, China. JAMA 2020; 323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johnston SL. Asthma and COVID‐19: is asthma a risk factor for severe outcomes? Allergy 2020; 75:1543–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy 2020; 75:1730–41. [DOI] [PubMed] [Google Scholar]
- 46. Licari A, Votto M, Brambilla I, et al Allergy and asthma in children and adolescents during the COVID outbreak: what we know and how we could prevent allergy and asthma flares? Allergy 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mahdavinia M, Foster KJ, Jauregui E, et al Asthma prolongs intubation in COVID‐19. J Allergy Clin Immunol Pract 2020; S2213–2198:30476–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Khawaja AP, Warwick AN, Hysi PG, Kastner A, Dick A, Khaw PT, et alAssociations with COVID‐19 hospitalisation amongst 406,793 adults: the UK Biobank prospective cohort study. medRxiv preprint 2020; 10.1101/2020.05.06.20092957. [DOI]
- 49. Williamson E, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE.The OpenSAFELY Collaborative. OpenSAFELY: factors associated with COVID‐19‐related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv preprint 2020; 10.1101/2020.05.06.20092999. [DOI]
- 50. Morais‐Almeida M, Barbosa MT, Sousa CS, Aguiar R, Bousquet J. Update on asthma prevalence in severe COVID‐19 patients. Allergy 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peters MC, Sajuthi S, Deford P, et al COVID‐19 Related Genes in Sputum Cells in Asthma: Relationship to Demographic Features and Corticosteroids. Am J Respir Crit Care Med 2020; 202:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. HLH Across Speciality Collaboration UK. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vaninov N. In the eye of the COVID‐19 cytokine storm. Nat Rev Immunol 2020; 20:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Han M, Rajput C, Hong JY, Lei J, Hinde JL, Wu Q, et al The Innate Cytokines IL‐25, IL‐33, and TSLP Cooperate in the Induction of Type 2 Innate Lymphoid Cell Expansion and Mucous Metaplasia in Rhinovirus‐Infected Immature Mice. J Immunol 2017; 199:1308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saravia J, You D, Shrestha B, Jaligama S, Siefker D, Lee GI, et al Respiratory Syncytial Virus Disease Is Mediated by Age‐Variable IL‐33. PLoS Pathog 2015; 11:e1005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hong JY, Bentley JK, Chung Y, Lei J, Steenrod JM, Chen Q, et al Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL‐25 and type 2 innate lymphoid cells. J Allergy Clin Immunol 2014; 134:429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stier MT, Bloodworth MH, Toki S, Newcomb DC, Goleniewska K, Boyd KL, et al Respiratory syncytial virus infection activates IL‐13‐producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J Allergy Clin Immunol 2016; 138:e811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Owczarczyk AB, Schaller MA, Reed M, Rasky AJ, Lombard DB, Lukacs NW. Sirtuin 1 Regulates Dendritic Cell Activation and Autophagy during Respiratory Syncytial Virus‐Induced Immune Responses. J Immunol 2015; 195:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schuler CF, Malinczak CA, Best SKK, Morris SB, Rasky AJ, Ptaschinski C, et al Inhibition of uric acid or IL‐1β ameliorates respiratory syncytial virus immunopathology and development of asthma. Allergy 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mahmutovic Persson I, Menzel M, Ramu S, Cerps S, Akbarshahi H, Uller L. IL‐1β mediates lung neutrophilia and IL‐33 expression in a mouse model of viral‐induced asthma exacerbation. Respir Res 2018; 19:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, Hershenson MB, et al IL‐17‐induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol 2011; 179:248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Malinczak CA, Fonseca W, Rasky AJ, Ptaschinski C, Morris S, Ziegler SF, et al Sex‐associated TSLP‐induced immune alterations following early‐life RSV infection leads to enhanced allergic disease. Mucosal Immunol 2019; 12:969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hashimoto K, Durbin JE, Zhou W, Collins RD, Ho SB, Kolls JK, et al Respiratory syncytial virus infection in the absence of STAT 1 results in airway dysfunction, airway mucus, and augmented IL‐17 levels. J Allergy Clin Immunol 2005; 116:550–7. [DOI] [PubMed] [Google Scholar]
- 64. Lukacs NW, Smit JJ, Mukherjee S, Morris SB, Nunez G, Lindell DM. Respiratory virus‐induced TLR7 activation controls IL‐17‐associated increased mucus via IL‐23 regulation. J Immunol 2010; 185:2231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Laza‐Stanca V, Stanciu LA, Message SD, Edwards MR, Gern JE, Johnston SL. Rhinovirus replication in human macrophages induces NF‐kappaB‐dependent tumor necrosis factor alpha production. J Virol 2006; 80:8248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ilarraza R, Wu Y, Skappak CD, Ajamian F, Proud D, Adamko DJ. Rhinovirus has the unique ability to directly activate human T cells in vitro. J Allergy Clin Immunol 2013; 131:395–404. [DOI] [PubMed] [Google Scholar]
- 67. Graser A, Ekici AB, Sopel N, Melichar VO, Zimmermann T, Papadopoulos NG, et al Rhinovirus inhibits IL‐17A and the downstream immune responses in allergic asthma. Mucosal Immunol 2016; 9:1183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aab A, Wirz O, van de Veen W, Söllner S, Stanic B, Rückert B, et al Human rhinoviruses enter and induce proliferation of B lymphocytes. Allergy 2017; 72:232–43. [DOI] [PubMed] [Google Scholar]
- 69. Venkataraman A, Bassis CM, Beck JM, Young VB, Curtis JL, Huffnagle GB, et al Application of a neutral community model to assess structuring of the human lung microbiome. MBio 2015; 6:e02284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rogers GB, Shaw D, Marsh RL, Carroll MP, Serisier DJ, Bruce KD. Respiratory microbiota: addressing clinical questions, informing clinical practice. Thorax 2015; 70:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Avadhanula V, Rodriguez CA, Devincenzo JP, Wang Y, Webby RJ, Ulett GC, et al Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species‐ and cell type‐dependent manner. J Virol 2006; 80:1629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang JH, Kwon HJ, Jang YJ. Rhinovirus enhances various bacterial adhesions to nasal epithelial cells simultaneously. Laryngoscope 2009; 119:1406–11. [DOI] [PubMed] [Google Scholar]
- 73. Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, et al Sustained desensitization to bacterial Toll‐like receptor ligands after resolution of respiratory influenza infection. J Exp Med 2008; 205:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ni K, Li S, Xia Q, Zang N, Deng Y, Xie X, et al Pharyngeal microflora disruption by antibiotics promotes airway hyperresponsiveness after respiratory syncytial virus infection. PLoS One 2012; 7:e41104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang J, Li F, Sun R, Gao X, Wei H, Li LJ, et al Bacterial colonization dampens influenza‐mediated acute lung injury via induction of M2 alveolar macrophages. Nat Commun 2013; 4:2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bousquet J, Jeffery PK, Busse WW, Johnson M, Asthma VAM. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000; 161:1720–45. [DOI] [PubMed] [Google Scholar]
- 77. Holgate ST. Rhinoviruses in the pathogenesis of asthma: the bronchial epithelium as a major disease target. J Allergy Clin Immunol 2006; 118:587–90. [DOI] [PubMed] [Google Scholar]
- 78. Looi K, Buckley AG, Rigby PJ, Garratt LW, Iosifidis T, Zosky GR, et al Effects of human rhinovirus on epithelial barrier integrity and function in children with asthma. Clin Exp Allergy 2018; 48:513–24. [DOI] [PubMed] [Google Scholar]
- 79. Kast JI, McFarlane AJ, Głobińska A, Sokolowska M, Wawrzyniak P, Sanak M, et al Respiratory syncytial virus infection influences tight junction integrity. Clin Exp Immunol 2017; 190:351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bossios A, Psarras S, Gourgiotis D, Skevaki CL, Constantopoulos AG, Saxoni‐Papageorgiou P, et al Rhinovirus infection induces cytotoxicity and delays wound healing in bronchial epithelial cells. Respir Res 2005; 6:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Edwards MR, Strong K, Cameron A, Walton RP, Jackson DJ, Johnston SL. Viral infections in allergy and immunology: How allergic inflammation influences viral infections and illness. J Allergy Clin Immunol 2017; 140:909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Altman MC, Reeves SR, Parker AR, Whalen E, Misura KM, Barrow KA, et al Interferon response to respiratory syncytial virus by bronchial epithelium from children with asthma is inversely correlated with pulmonary function. J Allergy Clin Immunol 2018; 142:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Patel DA, You Y, Huang G, Byers DE, Kim HJ, Agapov E, et al Interferon response and respiratory virus control are preserved in bronchial epithelial cells in asthma. J Allergy Clin Immunol 2014; 134:e1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhu J, Message SD, Mallia P, Kebadze T, Contoli M, Ward CK, et al Bronchial mucosal IFN‐α/β and pattern recognition receptor expression in patients with experimental rhinovirus‐induced asthma exacerbations. J Allergy Clin Immunol 2019; 143:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Edwards MR, Regamey N, Vareille M, Kieninger E, Gupta A, Shoemark A, et al Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol 2013; 6:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gill MA, Liu AH, Calatroni A, Krouse RZ, Shao B, Schiltz A, et al Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol 2018; 141:e1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Akbarshahi H, Menzel M, Ramu S, Mahmutovic Persson I, Bjermer L, Uller L. House dust mite impairs antiviral response in asthma exacerbation models through its effects on TLR3. Allergy 2018; 73:1053–63. [DOI] [PubMed] [Google Scholar]
- 88. Kicic A, Stevens PT, Sutanto EN, Kicic‐Starcevich E, Ling KM, Looi K, et al Impaired airway epithelial cell responses from children with asthma to rhinoviral infection. Clin Exp Allergy 2016; 46:1441–55. [DOI] [PubMed] [Google Scholar]
- 89. Ravanetti L, Dijkhuis A, Dekker T, Sabogal Pineros YS, Ravi A, Dierdorp BS, et al IL‐33 drives influenza‐induced asthma exacerbations by halting innate and adaptive antiviral immunity. J Allergy Clin Immunol 2019; 143:e1316. [DOI] [PubMed] [Google Scholar]
- 90. Lynch JP, Werder RB, Simpson J, Loh Z, Zhang V, Haque A, et al Aeroallergen‐induced IL‐33 predisposes to respiratory virus‐induced asthma by dampening antiviral immunity. J Allergy Clin Immunol 2016; 138:1326–37. [DOI] [PubMed] [Google Scholar]
- 91. Jurak LM, Xi Y, Landgraf M, Carroll ML, Murray L, Upham JW. Interleukin 33 Selectively Augments Rhinovirus‐Induced Type 2 Immune Responses in Asthmatic but not Healthy People. Front Immunol 2018; 9:1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Werder RB, Zhang V, Lynch JP, Snape N, Upham JW, Spann K, et al Chronic IL‐33 expression predisposes to virus‐induced asthma exacerbations by increasing type 2 inflammation and dampening antiviral immunity. J Allergy Clin Immunol 2018; 141:e1609. [DOI] [PubMed] [Google Scholar]
- 93. Zhu J, Message SD, Qiu Y, Mallia P, Kebadze T, Contoli M, et al Airway inflammation and illness severity in response to experimental rhinovirus infection in asthma. Chest 2014; 145:1219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nikonova A, Khaitov M, Jackson DJ, Traub S, Trujillo‐Torralbo MB, Kudlay DA, et al M1‐like macrophages are potent producers of anti‐viral interferons and M1‐associated marker‐positive lung macrophages are decreased during rhinovirus‐induced asthma exacerbations. EBioMedicine 2020; 54:102734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kelly BT, Grayson MH. Immunoglobulin E, what is it good for? Ann Allergy Asthma Immunol 2016; 116:183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kumar A, Grayson MH. The role of viruses in the development and exacerbation of atopic disease. Ann Allergy Asthma Immunol 2009; 103:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dakhama A, Lee YM, Ohnishi H, Jing X, Balhorn A, Takeda K, et al Virus‐specific IgE enhances airway responsiveness on reinfection with respiratory syncytial virus in newborn mice. J Allergy Clin Immunol 2009; 123:e135. [DOI] [PubMed] [Google Scholar]
- 98. Dakhama A, Park JW, Taube C, Chayama K, Balhorn A, Joetham A, et al The role of virus‐specific immunoglobulin E in airway hyperresponsiveness. Am J Respir Crit Care Med 2004; 170:952–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available.


