To the Editor,
The current pandemic of the novel SARS‐CoV‐2 infection has affected over 10 million humans around the planet. The clinical manifestations of coronavirus disease 2019 (COVID‐19) are diverse, ranging from asymptomatic or mild flu‐like symptoms to atypical pneumonia, severe respiratory distress syndrome, systemic inflammation, immune dysregulation, and coagulopathy.
Inborn errors of immunity (IEI) are a heterogeneous group of more than 430 rare congenital disorders with increased susceptibility to infection, autoimmunity, atopy, hyperinflammation, and cancer. Autosomal recessive ARPC1B deficiency is an actinopathy, as are DOCK8 deficiency and the Wiskott‐Aldrich syndrome. Defective actin polymerization affects hematopoietic cells, impairing their migration and immunological synapse 1 , which results in a combined immune deficiency characterized by leukocytosis, eosinophilia, platelet abnormalities, and hypergammaglobulinemia, and clinically, by eczema and food allergy; infections caused by bacteria, fungi, and viruses; vasculitis; and bleeding diathesis 2 . Here, we describe a male infant patient with known ARPC1B deficiency, who was hospitalized for COVID‐19 pneumonia and improved without requiring intensive care or mechanical ventilation. Informed consent through protocols approved by the institutional review boards of the National Institute of Pediatrics was obtained from the patient’s family.
An 8‐month‐old male infant was brought to the emergency department with high‐grade fever. His family history is remarkable for one brother who died as a newborn from intracranial bleeding and an 11‐year‐old sister with the same genetic defect and decreased proliferative response to mitogens, who underwent hematopoietic stem cell transplantation twice without success, and is currently on antimycobacterial treatment, antimicrobial prophylaxis, and regular subcutaneous immunoglobulin. The patient was first seen at age 1 month for eczema and rectal bleeding attributed to cow milk protein allergy. At age 4 months, he developed bronchiolitis caused by respiratory syncytial virus (RSV) and oral candidiasis. Laboratory workup revealed leukocytosis (17 500‐33 600/mm3), eosinophilia (5600‐20 100/mm3), and a marginally high platelet count (467 000) with normal platelet volume, as well as high serum IgG (737 mg/dL) and IgA (165 mg/dL), with normal IgM (37.7 mg/dL). Lymphocyte subsets showed slightly low CD3+ (2.315 cells), normal CD4+ (23%, 1974), and low CD8+ (3%, 257 cells). B cells and NK cells were elevated at 48% (4116 cells) and 15% (1286 cells), respectively. Whole exome sequencing identified a homozygous 46‐base pair deletion in exon 8 of ARPC1B (chr7: 99,392,784 hg38; p.Glu300fs).
Upon his arrival to the emergency department, he was febrile with tachycardia and signs of septic shock, requiring rapid fluid resuscitation. He showed no respiratory or gastrointestinal signs, although he also had a post‐traumatic ulcerated lesion under the tongue with dark discoloration, which raised a concern for fungal infection. Intravenous antibiotics (ciprofloxacin) with antifungal coverage were started within the first hour and a dose of intravenous immunoglobulin (IVIG) at 1 g/kg. Blood counts revealed leukocytosis, neutrophilia, and mild eosinophilia without lymphopenia, while platelets were initially found within normal limits. A day later, blood culture had grown Pseudomonas aeruginosa.
During his second day of hospitalization, the patient persisted febrile, tachycardic, and tachypneic, with oxygen desaturation into the low 80s. Chest X‐ray showed non‐specific bilateral interstitial opacities in the perihilar regions (Figure 1). Real‐time polymerase chain reaction (RT‐PCR) for SARS‐CoV‐2, an oral swab taken upon admission, came back positive, and he was then transferred to a COVID‐19 isolation area. There was no known contact with another positive COVID‐19 case.
FIGURE 1.
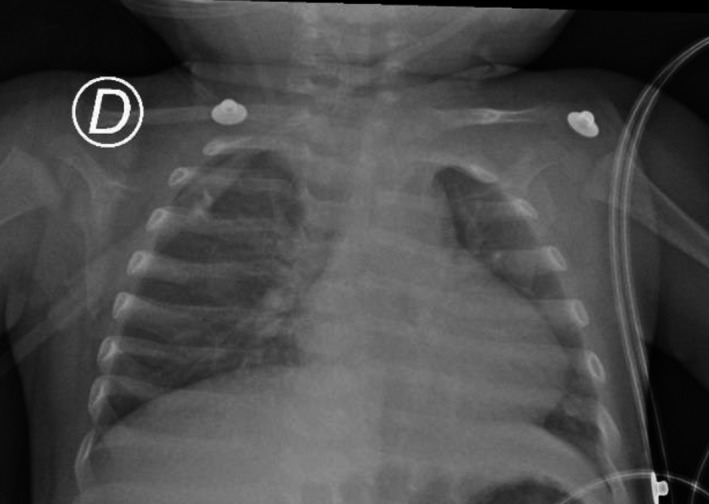
Bilateral interstitial infiltrates with perihilar predominance
The potassium hydroxide (KOH) test for oral thrush was negative for yeast cells, after which amphotericin was switched to fluconazole. Supplemental oxygen was discontinued on day 6 of hospitalization, when mild thrombocytopenia and a prolonged thromboplastin time (aPTT) (but normal fibrinogen and ferritin serum levels) were reported (see Table 1). After completing 14 days of antimicrobial treatment, the patient was discharged without ever requiring intensive care unit admission or mechanical ventilation. Specific serum antibodies to SARS‐CoV‐2 were assessed 8 weeks after the positive RT‐PCR test and came back negative.
TABLE 1.
Blood counts and acute‐phase reactants on admission and on day 5
| Hospital admission (day 0) | Day 5 of hospital admission | |
|---|---|---|
| Hb | 10.4 mg/dL | 12.9 mg/dL |
| Leukocytes | 40 300 cells/μL | 9800 cells/μL |
| Neutrophils | 30 100 cells/μL | 3800 cells/μL |
| Lymphocytes | 5400 cells/μL | 2400 cells/μL |
| Eosinophils | 600 cells/μL | 2000 cells/μL |
| Platelets | 165 000 | 109 000 |
| C‐reactive protein | Not available | Not available |
| D‐dimer | Not available | Not available |
| Fibrinogen | Not available | 265 mg/dL |
| Triglycerides | Not available | 219 mg/dL |
| Ferritin | Not available | 268 ng/mL |
The behavior of COVID‐19 in patients with IEI might help dissect the immune response to SARS‐CoV‐2. A few cases of adults with COVID‐19 and predominantly antibody deficiencies have been reported 3 , 4 ; some of them developed acute respiratory distress syndrome (ARDS), while some had a milder course of illness. Based on what we know, innate immune defects in genes involved in type 1 interferon response (such as IRF7, IRF9, TLR3) are the most likely candidates to result in severe disease and death in patients with flu‐like virus infection 5 . In a few cases of fatal influenza A (H1N1), variants in genes associated with familial hemophagocytic lymphohistiocytosis (FHL) and a decreased cytolytic function of NK cells were also reported 6 .
Our patient was on monthly supplemental IVIG treatment, and he received an additional dose during his hospital stay. This, and his young age, might have ameliorated the clinical course 7 . He had a favorable evolution, despite the known susceptibility to viral infection and immune dysregulation in ARPC1B‐deficient patients 1 . There were no signs of severe infection, ARDS, and hyperinflammation or of “cytokine storm” unleashed by SARS‐CoV‐2. Despite him having a combined immune deficiency, our patient fully recovered without the need of additional supportive measures other than IVIG, supplemental oxygen, and antibiotic treatment directed against the documented bacteremia.
Although pediatric cases of COVID‐19 are fewer compared to adults, some severe presentations and deaths among children have been reported. The presence of a restricted repertoire of IgG (since infants have no previous exposure to coronaviruses) might play a role in the better outcome seen in pediatric patients. Antibody‐dependent enhancement has been implicated in the development of severe COVID‐19 in the elderly 8 . Additionally, lung cells from children and women show a lower expression of membrane‐bound ACE‐2, which may also be protective against severe pneumonia.
Conceivably, some immune defects could protect patients with certain IEIs from mounting a full uncontrolled inflammatory response against SARS‐CoV‐2 9 . The cytoskeleton is a regulator of gene transcription, coupling cell mechanics with the activity of NF‐κB. RNA betacoronaviruses are thought to alter the cytoskeleton architecture to facilitate viral replication and output 10 . Thus, ARPC1B deficiency and other actinopathies might limit SARS‐CoV‐2 replication. Furthermore, Th2 cytokines modulate ACE2 (angiotensin‐converting enzyme 2) and TMPRSS2 expression in airway epithelial cells 11 , and children with allergies (asthma and/or allergic rhinitis) have a lower expression of ACE2 12 . Patients with ARPC1B deficiency often have allergic diseases; their Th2‐biased response could help explain the milder presentation seen in our patient. Insights from protective mechanisms in children, with and without certain immune defects, could facilitate the identification of therapeutic targets.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Lina Maria Castano‐Jaramillo: Data curation (equal); investigation (equal); writing‐original draft (lead); writing‐review and editing (equal). Marco Antonio Yamazaki‐Nakashimada: Investigation (equal); supervision (equal); writing‐review and editing (equal). Selma Cecilia Scheffler Mendoza: Data curation (equal); supervision (equal); writing‐review and editing (equal). Juan Carlos Bustamante‐Ogando: Writing‐review and editing (equal). Sara Elva Espinosa‐Padilla: Supervision (supporting); validation (supporting); visualization (supporting); writing‐review and editing (supporting). Saul O. Lugo Reyes: Conceptualization (lead); supervision (lead); writing‐original draft (lead); writing‐review and editing (equal).
ETHICAL APPROVAL
The patient and his family gave written informed consent for the diagnostic procedures and for publication of the case report.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13322.
Marco Antonio Yamazaki‐Nakashimada and Saul O. Lugo Reyes authors contributed equally.
REFERENCES
- 1. Randzavola LO, Strege K, Juzans M, et al. Loss of ARPC1B impairs cytotoxic T lymphocyte maintenance and cytolytic activity. J Clin Invest. 2019;129(12):5600‐5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuijpers TW, Tool ATJ, van der Bijl I, et al. Combined immunodeficiency with severe inflammation and allergy caused by ARPC1B deficiency. J Allergy Clin Immunol. 2017;140(1):273‐277.e10. [DOI] [PubMed] [Google Scholar]
- 3. Fill L, Hadney L, Graven K, Persaud R, Hostoffer R. The clinical observation of a patient with common variable immunodeficiency diagnosed as having coronavirus disease 2019. Ann Allergy Asthma Immunol. 2020;125:112‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soresina A, Moratto D, Chiarini M, et al. Two X‐linked agammaglobulinemia patients develop pneumonia as COVID‐19 manifestation but recover. Pediatr Allergy Immunol. 2020;31:565‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casanova J‐L, Su HC. A global effort to define the human genetics of protective immunity to SARS‐CoV‐2 infection. Cell. 2020;181:1194‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schulert GS, Zhang M, Fall N, et al. Whole‐exome sequencing reveals mutations in genes linked to hemophagocytic lymphohistiocytosis and macrophage activation syndrome in fatal cases of H1N1 influenza. J Infect Dis. 2016;213(7):1180‐1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aljaberi R, Wishah K. Positive outcome in a COVID‐19 patient with common variable immunodeficiency after IVIG. Ann Allergy Asthma Immunol. 2020;S1081‐1206(20)30402‐6. 10.1016/j.anai.2020.06.006. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peron JPS, Nakaya H. Susceptibility of the elderly to SARS‐CoV‐2 infection: ACE‐2 overexpression, shedding, and antibody‐dependent Enhancement (ADE). Clinics. 2020;75:e1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matricardi PM, Dal Negro RW, Nisini R. The first, holistic immunological model of COVID‐19: implications for prevention, diagnosis, and public health measures. Pediatr Allergy Immunol. 2020;31:454‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uhler C, Shivashankar GV. Mechano‐genomic regulation of coronaviruses and its interplay with ageing. Nature Rev Mol Cell Biol. 2020;21(5):247‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimura H, Francisco D, Conway M, et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146(1):80‐88.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson DJ, Busse WW, Bacharier LB, et al. Association of respiratory allergy, asthma, and expression of the SARS‐CoV‐2 receptor ACE2. J Allergy Clin Immunol. 2020;146:203‐206.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]


