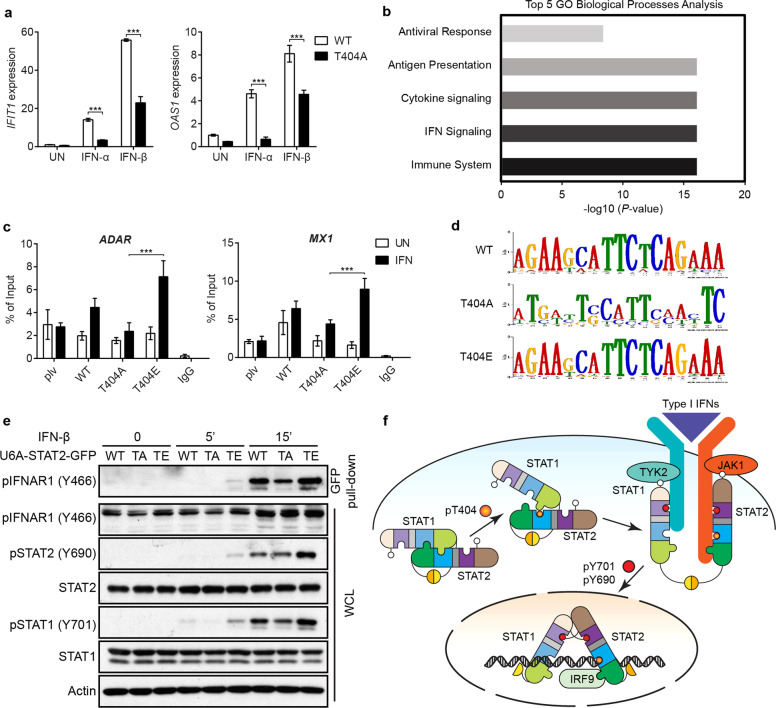
Fig. 2. Phosphorylation of STAT2 on T404 expedites the tyrosine phosphorylation of STAT1 and STAT2, and enhances the DNA-binding activity of ISGF3.
a U6A cells expressing WT or T404A STAT2 were treated with IFN-α or IFN-β (100 IU/ml). Cells were harvested after 4 h and total RNAs were analyzed by qRT-PCR. b U6A cells expressing WT or T404A STAT2 were treated with IFN-β for 0, 4, 8, or 24 h. Total RNAs were analyzed by using an Illumina HumanHT-12 v4 Expression BeadChip array. The average signal for each probe was used to determine expression levels. Genes with detection P values greater than 0.01 in the untreated or treated cells were excluded from the analysis. Inductions of less than 2-fold were not scored. A gene ontology (GO) analysis was performed of genes differentially expressed between U6A cells expressing T404A or WT STAT2, stimulated with IFN-β for 4, 8, or 24 h. c The occupancy of the ADAR1 and MX1 promoters by IRF9 in U6A cells expressing WT, T404A, or T404E STAT2, treated with IFN-β (100 IU/mL), was assayed by ChIP, using anti-IRF9. d Samples from c were analyzed by ChIP-seq. The top 100 segments of each sample were used as inputs. Motifs were discovered by MEME v5.0.3. e U6A cells expressing GFP-tagged WT, T404A, or T404E STAT2 were treated with IFN-β (100 IU/mL) for 0, 5, or 15 min. Whole-cell lysates were used for GFP pull-down and were analyzed by western blot. f Working model shows that, T404 phosphorylation, by destabilizing the U-STAT1-U-STAT2 anti-parallel dimer, increases the affinity of STAT2 for activated IFNAR1, expedites the tyrosine phosphorylation of both STAT2 and STAT1, and enhances the affinity of ISGF3 for ISREs. Data are shown as means ± SEM from three independent experiments. P values were calculated using the paired ratio t-test (***P < 0.001).