Figure 1. A disulfide tethering screen identifies covalent BFL-1 inhibitor molecules that disrupt BH3-binding activity.
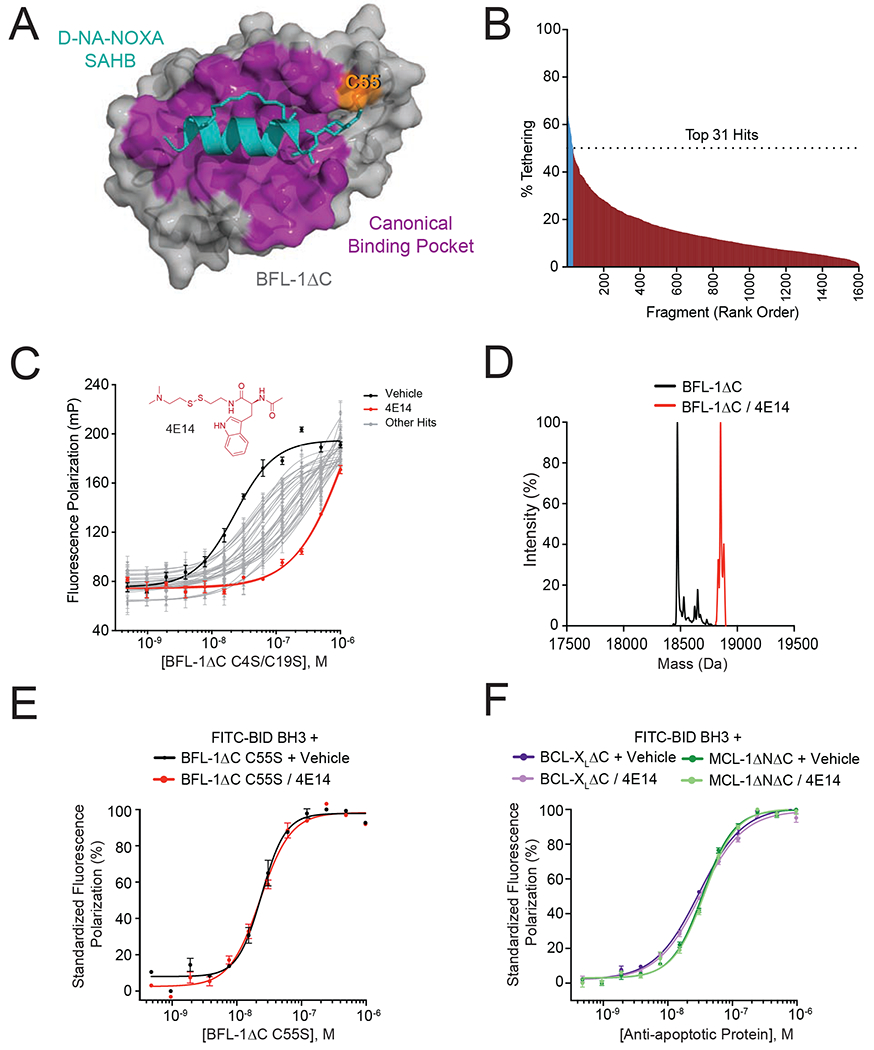
(A) Crystal structure of the complex between anti-apoptotic BFL-1ΔC (grey) and a cysteine-reactive stapled NOXA BH3 peptide (cyan) (PDB: 5WHH), highlighting the site of covalent attachment to C55 (orange) and interaction at the canonical BH3-binding groove (purple).
(B) A disulfide tethering screen of 1600 disulfide fragments against BFL-1ΔC C4S/C19S yielded 31 fragments with percent tethering of at least two standard deviations above the mean, as measured by mass spectrometry.
(C) Fluorescence polarization (FP) assays comparing the relative capacity of the 31 identified fragments to compete with FITC-BID BH3 peptide for interaction at the BFL-1ΔC C4S/C19S groove. 4E14 emerged as the most potent competitive inhibitor of the FITC-BID BH3/BFL-1 interaction (EC50: FITC-BID BH3/BFL-1, 23 nM; FITC-BID BH3/BFL-1–4E14, 1.3 μM). Data are mean ± S.D. for experiments performed in technical triplicate and repeated two times with independent preparations of ligand and protein with similar results.
(D) Complete derivatization of His6-BFL-1ΔC by 4E14, as measured by intact mass spectrometry (molecule:protein, 20:1). Left peak (black): 18,473 Da (BFL-1 minus Met plus H2O); right peak (red): 18,777 Da (M+304).
(E-F) FP analysis evaluating the capacity of 4E14 to compete with FITC-BID BH3 for interaction with BFL-1ΔC C55S (E) and BCL-XLΔC and MCL-1ΔNΔC (F). Data are mean ± S.E.M. for experiments performed in technical triplicate and repeated two times with independent preparations of ligand and protein with similar results.
See also Table S1.
