Abstract
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) is responsible for an unprecedented worldwide pandemic that has severely impacted the United States. As the pandemic continues, a growing body of evidence suggests that infected patients may develop significant coagulopathy with resultant thromboembolic complications including deep vein thrombosis, pulmonary embolism, myocardial infarction, and ischemic stroke. However, this data is limited and comes from recent small case series and observational studies on stroke types, mechanisms, and outcomes.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 Furthermore, evidence on the role of therapeutic anticoagulation in SARS-CoV-2 infected patients with elevated inflammatory markers, such as D-dimer, is also limited. We report the case of a middle-aged patient who presented with a large vessel ischemic stroke likely resulting from an underlying inflammatory response in the setting of known novel coronavirus infection (COVID-19). Histopathologic analysis of the patient's ischemic brain tissue revealed hypoxic neurons, significant edema from the underlying ischemic insult, fibrin thrombi in small vessels, and fibroid necrosis of the vascular wall without any signs of vasculature inflammation. Brain biopsy was negative for the presence of SARS-CoV-2 RNA (RT-PCR assay). Along with a growing body of literature, our case suggests that cerebrovascular thromboembolic events in COVID-19 infection may be related to acquired hypercoagulability and coagulation cascade activation due to the release of inflammatory markers and cytokines, rather than virus-induced vasculitis. Further studies to investigate the mechanism of cerebrovascular thromboembolic events and their prevention is warranted.
Key Words: Ischemic stroke, Inflammatory conditions, COVID-19, Corona virus, SARS-CoV-2 RNA, cerebrovascular disease, hemorrhagic stroke, cerebral sinus thrombosis, vasculitis, anticoagulation, thrombotic conditions, thromboembolic conditions
Introduction
The first retrospective study from China during the current COVID-19 pandemic reported a higher incidence of cerebrovascular disease and decreased functional outcomes for those admitted for COVID-19 infection.3 A recently published article from New York reported ischemic stroke cases in a young-to-middle-age population without apparent risk factors.1 One theory of COVID-19 associated hypercoagulability proposes that SARS-CoV-2 facilitates recruitment of inflammatory cells in blood vessels, which leads to the release of inflammatory markers and cytokines that subsequently activate the coagulation cascade.15 However, no causal relationship has been formally established between COVID-19 infection and cerebrovascular injury. In order to aid in the study of SARS-CoV-2 associated cerebrovascular injuries, we have reviewed the literature and compiled a series of studies reporting the incidence of cerebrovascular disease in COVID-19 infected patients (Table 1 ). Further, we describe the case of a malignant ischemic stroke in a middle-aged adult admitted with COVID-19 infection with associated histopathological findings, which represents previously unreported findings.
Table 1.
Case Series and Observational Studies of Cerebrovascular Disease (CVD) Manifestations from COVID-19
| Authors | Type of study | Sample Size | CVD cases | |
|---|---|---|---|---|
| 1. | Oxley et al. | Case series | 5 | 5 AIS |
| 2. | Li et al. | Retrospective, observational study | 219 | 10 AIS & 1 ICH |
| 3. | Mao et al. | Retrospective, observational study | 214 | 4 AIS & 1 ICH |
| 4. | Klok et al. | Retrospective, observational study | 184 | 3 AIS |
| 5. | Helms et al. | Retrospective, observational study | 58 | 3 AIS |
| 6. | Avula et al. | Case series | 4 | 4 AIS |
| 7. | Beyrouti et al. | Case series | 6 | 6 AIS and 2 CVT |
| 8. | Morassi et al. | Case series | 6 | 4 AIS and 2 ICH |
| 9. | Varatharaj et al. | Surveillance study | 125 | 57 AIS, 9 ICH, and 1 CNS vasculitis. |
| 10. | Yaghi et al. | Retrospective, cohort study | 3,556 | 32 AIS |
| 11. | Lodigiani et al. | Retrospective, cohort study | 388 | 9 AIS |
| 12. | Helms et al. | Prospective, cohort study | 150 | 2 AIS |
| 13. | Klok et al. | Retrospective, observational study | 184 | 5 AIS |
| 14. | Abdulkadir et al. | Case series | 4 | 4 AIS |
Abbreviation: AIS: acute ischemic stroke, ICH: intracranial hemorrhage, CVT: cerebral sinus thrombosis, CNS: central nervous system
Case
A 48-year-old right-handed male with a prior history of obesity, hypertension, hyperlipidemia, right below-the-knee amputation since childhood, history of drug abuse, and gout presented with one-week of dyspnea and cough. Two weeks prior to his presentation, he had been exposed to a COVID-19-positive patient in a skilled nursing facility where had been admitted for physical therapy following a gout flare.
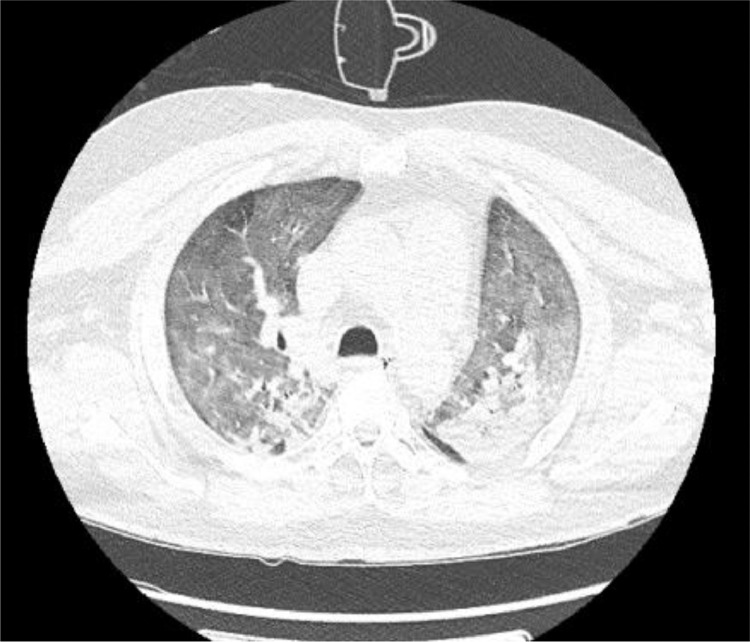
Upon arrival to the emergency department, he was febrile to 100.5°F with a heart rate of 98 bpm, blood pressure of 124/77 mmHg, and oxygen saturation of 92% via non-rebreather mask. He was admitted to the medical intensive care unit for acute hypoxic respiratory failure without any focal neurological signs. On day 2 of his hospitalization, SARS-CoV-2 RT-PCR testing returned positive. Chest radiograph demonstrated extensive airspace opacities throughout the bilateral lungs with left-sided consolidation, likely reflective of multifocal pneumonia. Later in the course, a CT of the thorax confirmed the presence of bilateral confluent consolidation in the lower lobes with air bronchograms and diffuse ground-glass opacification in the apex of the left upper lobe and anterior right middle lobe (Figure 1 ). He was treated with high flow oxygen supplementation, antibiotics (cefepime and vancomycin), methylprednisolone, convalescent plasma, tocilizumab immunotherapy, and intermittent prone positioning. On day 3 of admission, his laboratory findings were significant for elevated C-reactive protein (4.19 mg/dl), fibrinogen level (> 1000 mg/dl), and D-dimer (1093 ng/ml) concerning for hypercoagulability and for which he was started on therapeutic low molecular weight heparin (LMWH) at 1 mg/kg dosing every 12 hours for prophylaxis of hypercoagulable state.
Figure 1.
CT of the thorax with findings suggestive of viral pneumonia with superimposed edema.
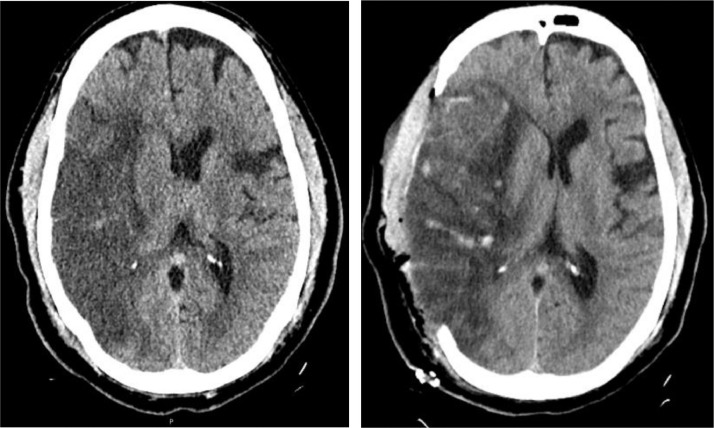
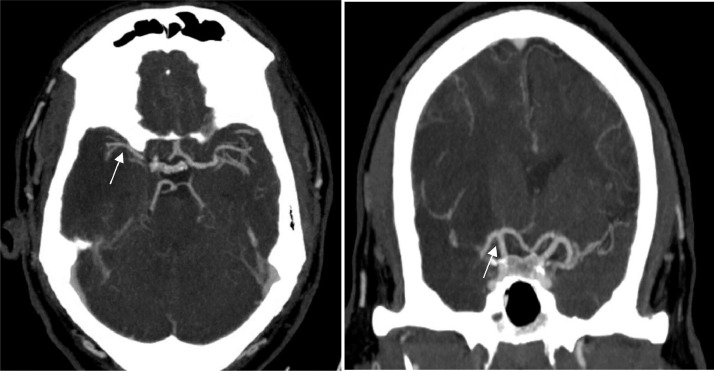
On day 4 of admission, he developed garbled speech and left-sided hemiplegia (Manual Muscle Testing Grading System 0 out of 5 scales) while on therapeutic dose of anticoagulation with Partial Thromboplastin Time (PTT) of 24 seconds. He had last been seen at neurologic baseline at 10:00 on day 3 of hospitalization, as recognition of earlier-onset mild dysarthria was masked by frequent sedative medication administration for episodic agitation. Upon evaluation, telemetry showed normal sinus rhythm, and the National Institutes of Health Stroke Scale (NIHSS) was 16 (scores range from 0 to 42, with higher numbers indicating greater stroke severity) for right gaze, dysarthria, left-sided neglect, and left-sided hemiplegia. Computed tomography (CT) of the head showed a large right middle cerebral artery (RMCA) infarct with an Alberta Stroke Program Early CT (ASPECT) Score of 3 and 5 mm of right to left midline shift (Figure 2 ). CT angiogram of the head and neck did not reveal a well-defined thrombus to suggest large vessel occlusion, though focal narrowing of the right MCA M1/M2 was noted (Figure 3 ). Further, CT angiography did not demonstrate evidence of vessel dissection or atherosclerotic plaques. He was not an intravenous tissue plasminogen activator (IV-tPA) candidate based on the time elapsed since last known normal and a low ASPECT score due to risk of hemorrhagic transformation. Therapeutic anticoagulation was discontinued at that time due to the risk of hemorrhage. Transthoracic echocardiography was negative for cardiac source of embolus, valvular dysfunction, regional wall motion abnormalities, or atrial septal defect. Lower extremity venous Doppler ultrasound did not demonstrate evidence of deep venous thrombosis. Urine toxicological screening for amphetamines, barbiturates, marijuana, cocaine, and phencyclidine was negative.
Figure 2.
Non-contrast CT scans of the head pre- and post-operatively. The image on the left demonstrates RMCA territorial infarct with midline shift and ventricular effacement prior to surgical intervention. The image on the right demonstrates infarcted brain parenchyma with herniation beyond the skull defect following right-sided decompressive hemicraniectomy.
Figure 3.
Contrast CT Angiogram of the head did not show RICA or RM1 segment occlusion. RMCA proximal segment was narrower compared to the contralateral side.
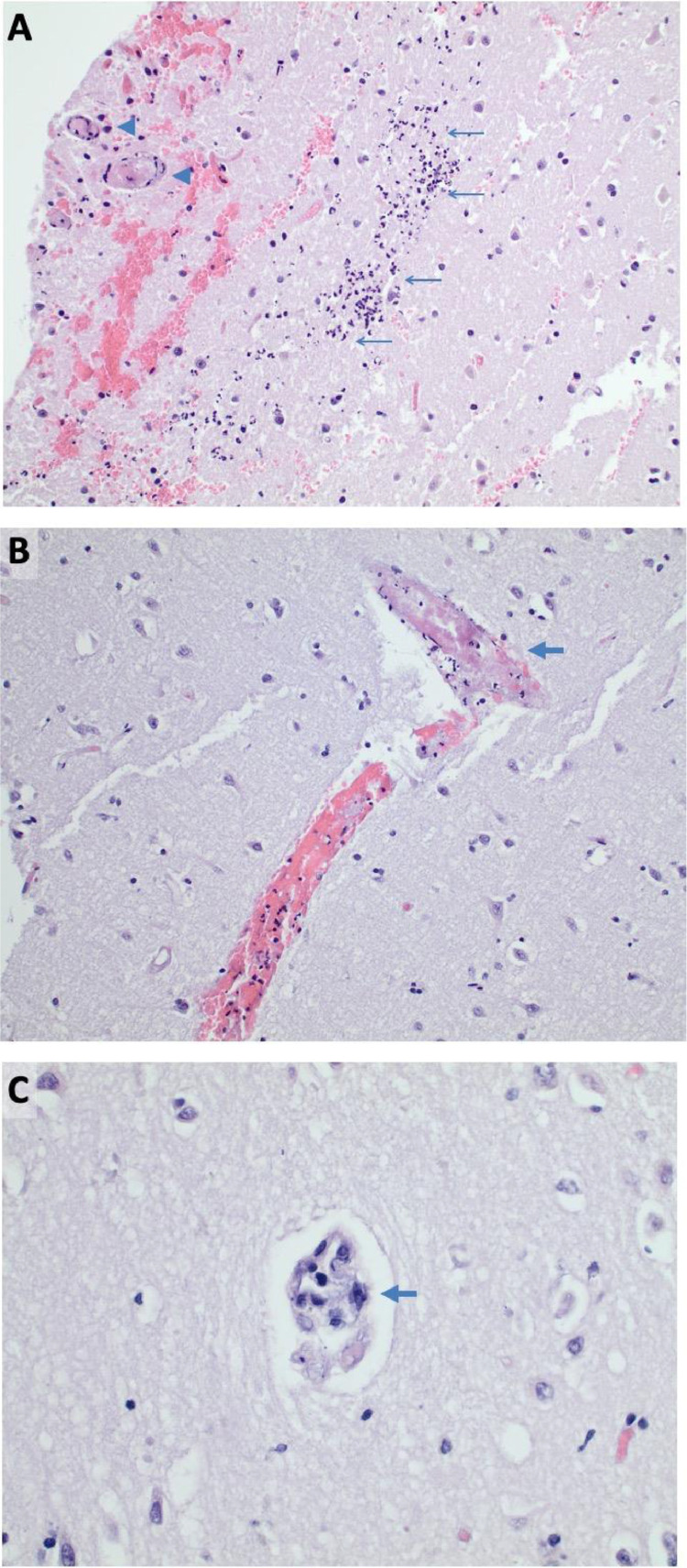
He was initially managed with osmotherapy in the setting of significant cytotoxic edema and resultant midline shift in the setting of large territorial cerebral infarction. Surveillance stability CT scan of the head obtained on post-stroke day 2 demonstrated progressive cerebral edema with leftward midline shift increased to 1.4 cm in size and concurrent right uncal herniation with mesencephalic compression despite aggressive medical management. Given his rapid radiographic progression and concern for declining mental status, he underwent decompressive hemicraniectomy (DHC) within 72 hours of stroke symptoms. Brain biopsy of right temporal lobe infarcted tissue was obtained that showed acute cerebritis characterized by marked perivascular and parenchymal neutrophilic infiltrates, associated acute hypoxic neurons and marked edema. Many small vessels showed fibrin thrombi, some with fibroid necrosis of the vascular wall, without any signs of vasculature inflammation. The sample also underwent RT-PCR assay for SARS-CoV-2 RNA with an eventual negative result (Figure 4 ). Eventually, the patient had worsening respiratory status and multifactorial shock. Given his grave prognosis, further goals of care were discussed with the palliative care team and family, and it was decided that he should be transitioned to comfort measures only.
Figure 4.
Histopathologic specimens of right temporal lobe necrotic tissue. A) Intermediate power microscopy (H&E 100x) demonstrates acute encephalitis (arrows) and microvascular thrombi (arrow heads) within the acutely infarcted area with background parenchymal edema. B) A thrombosed vessel away from the inflamed areas (arrow) (H&E 100x). C) A small vessel away from the inflamed area demonstrating marked endothelial reactive atypia (arrow) (H&E 400x).
Discussion
There have been numerous reports in the literature of an association between COVID-19 infection and thromboembolic complications, including ischemic stroke, during the current pandemic.4 , 16 , 17 One recent retrospective cohort study conducted in the New York metropolitan area revealed that, while the overall rate of imaging-confirmed ischemic stroke remained low in their sample, the rate of cryptogenic stroke in those infected with COVID-19 was significantly higher than that observed in both contemporary and historical control groups.10 While further study on etiology and management of stroke in this setting is required, there is speculation that these events may be related to acquired hypercoagulability. Our case of COVID-19 associated malignant cerebral infarct is the first to examine associated histological and biopsy findings, and joins an expanding body of literature with findings of COVID-19 related systemic inflammation leading to activation of the coagulation cascade and resulting in thromboembolic events. The histopathologic analysis of this specimen did not reveal evidence of primary CNS infection with the SARS-CoV-2 virus, suggesting that other vasculopathic factors, perhaps related to a systemic, inflammatory-mediated pro-thrombotic state, may have contributed to this event. Our patient's biopsy specimen did not show evidence of vasculitis, but rather demonstrated microthrombi within the vessel more consistent with a systemic inflammatory response mediated mechanism, probably related to the recorded elevated serum inflammatory markers, including D-dimer and fibrinogen.
This case is also noteworthy, as the patient developed an ischemic stroke in the setting of therapeutic anticoagulation (which was started for elevated D-dimer and fibrinogen levels). Previous studies have reported venous thromboembolism and ischemic stroke in approximately 20% and 3% of SARS-CoV-2 infected patients, respectively.18 Severely ill patients, particularly those admitted to intensive care units, were noted to have a higher risk of thrombotic events. The thrombotic risk remains elevated even with standard anticoagulation therapy, raising the question of optimal doses of and agents for anticoagulation, especially in the ischemic stroke cohort. To this end, PROTECT COVID (A Randomized Trial of Anticoagulation Strategies in COVID-19), is an on-going randomized clinical trial analyzing the safety and efficacy of therapeutic versus prophylactic anticoagulation in COVID-19 infection and elevated D-dimer patients.10 This case demonstrates the importance of further study of the mechanisms of cerebrovascular thromboembolic events and the efficacy of therapeutic anticoagulation in the setting of COVID-19 and its co-morbid inflammatory-mediated pro-thrombotic state.
Acknowledgments
Study concept and design: Patel, Pervez
Drafting of the manuscript: Patel, Kollar
Critical revision of the manuscript for valuable intellectual content: All authors.
Administrative, technical, or material support: All authors
Study supervision: Pervezt
Funding
This research received no specific grant from any funding agency in public, commercial, or not-for-profit sectors. The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Oxley TJ, Mocco J, Majidi S. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. New England Journal of Medicine. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Li M, Wang M. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke and Vascular Neurology. 2020 doi: 10.1136/svn-2020-000431. svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Jin H, Wang M. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA neurology. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok FA, Kruip MJHA, van der Meer NJM. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thrombosis Research. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J, Kremer S, Merdji H. Neurologic Features in Severe SARS-CoV-2 Infection. New England Journal of Medicine. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avula A, Nalleballe K, Narula N. COVID-19 presenting as stroke. Brain, behavior, and immunity. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyrouti R, Adams ME, Benjamin L. Characteristics of ischaemic stroke associated with COVID-19. Journal of neurology, neurosurgery, and psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morassi M, Bagatto D, Cobelli M. Stroke in patients with SARS-CoV-2 infection: case series. Journal of neurology. 2020;267:2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. The Lancet Psychiatry. [DOI] [PMC free article] [PubMed]
- 10.Yaghi S, Ishida K, Torres J. SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lodigiani C, Iapichino G, Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helms J, Tacquard C, Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive care medicine. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.TunÇ A, ÜnlÜbaŞ Y, Alemdar M, AkyÜz E. Coexistence of COVID-19 and acute ischemic stroke report of four cases. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2020;77:227–229. doi: 10.1016/j.jocn.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klok FA, Kruip MJHA, van der Meer NJM. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valderrama EV, Humbert K, Lord A, Frontera J, Yaghi S. Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Ischemic Stroke. Stroke. 2020;51:e124–e1e7. doi: 10.1161/STROKEAHA.120.030153. [DOI] [PubMed] [Google Scholar]
- 16.Lodigiani C, Iapichino G, Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin DO, Jensen A, Khan M. Arterial thromboembolic complications in COVID-19 in low-risk patients despite prophylaxis. British Journal of Haematology. 2020;190:e11–ee3. doi: 10.1111/bjh.16792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Ani F, Chehade S, Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thrombosis Research. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]