Table 2.
Optimization of ring closing metathesis on macrocycle precursors 8c and 8d, which contain Z alkenes.
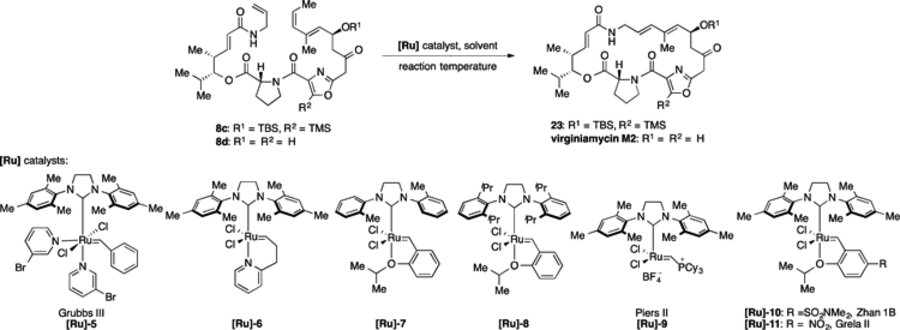 | |||||
|---|---|---|---|---|---|
| Entry | RCM precursor | Catalyst | Solvent | T(°C) | Yield |
| 1 | 8c | [Ru]-1 (20 mmol%) | Tol | 23 | -a |
| 2 | 8c | [Ru]-2 (20 mmol%) | Tol | 23 | 25%b |
| 3 | 8c | [Ru]-3 (20 mmol%) | Tol | 23 | -a |
| 4 | 8c | [Ru]-4 (20 mmol%) | Tol | 23 | 38%b |
| 5 | 8c | [Ru]-5 (20 mmol%) | Tol | 23 | -a |
| 6 | 8c | [Ru]-6 (20 mmol%) | Tol | 23 | -a |
| 7 | 8c | [Ru]-7 (20 mmol%) | Tol | 23 | 27%b |
| 8 | 8c | [Ru]-4 (8 mmol%) × 2 | Tol | 23 | 40%b |
| 9 | 8c | [Ru]-4 (8 mmol%) × 2 | BTF | 23 | 42%b |
| 10 | 8c | [Ru]-8 (8 mmol%) × 2 | BTF | 23 | 33%b |
| 11 | 8c | [Ru]-9 (8 mmol%) × 2 | BTF | 0 | -a |
| 12 | 8c | [Ru]-10 (8 mmol%) × 2 | BTF | 23 | 28%b |
| 13 | 8c | [Ru]-11 (8mmol%) × 2 | BTF | 23 | 49%c |
| 14 | 8d | [Ru]-11 (8 mmol%) × 2 | DCM | 23 | 72%c |
No product detected.
Yield determined by 1H NMR analysis of the crude reaction mixture using 1,4-dinitrobenzene as an internal standard.
isolated yield after column chromatography. BTF = benzotrifluoride, DCM = dichloromethane, Tol = toluene.
