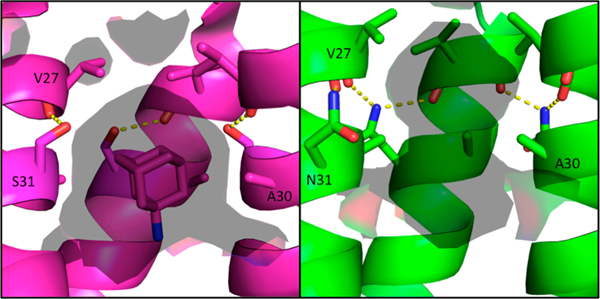
Figure 8.
Solvent-accessible surface area inside the M2 pore at the adamantane binding site. Amantadine-bound WT structure in the Inwardclosed conformation 6BKK (left, magenta) vs S31N Inwardclosed conformation (green, right); solvent-accessible surface area (calculated using a 1.4 Å probe) is shown as a transparent surface. In the WT channel, Ser31 forms an intrahelix hydrogen bond and partially faces the pore. In the S31N mutant, Asn31 faces the monomer–monomer interface, twisting the monomer helices such that Ala30 faces the center of the pore. This constricts and elongates the pore at the amantadine binding site for the S31N mutant M2 channel.