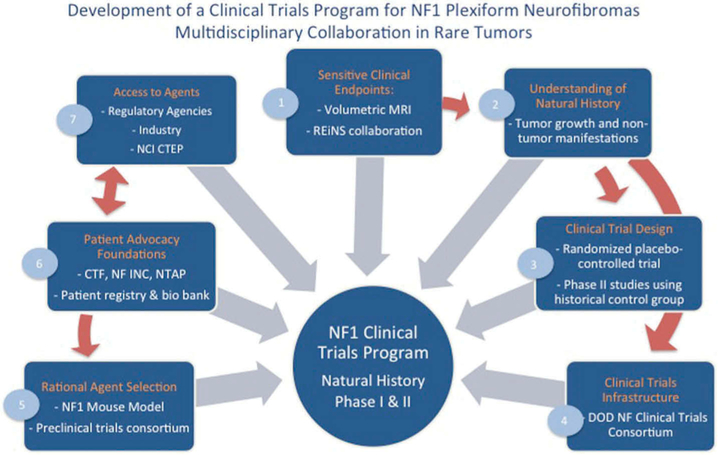
Figure 1. Development of a clinical trial program for NF1 plexiform neurofibromas: multidisciplinary collaboration in rare tumors.
- The development of volumetric MRI analysis of PN allowed sensitive and reproducible measurement of changes in PN size and is now recommended and used as a sensitive endpoint in most clinical trials directed at PN.[49,50] As more clinical trials for NF develop, the Response Evaluation in Neurofibromatosis and Schwannomatosis international working group was launched with the goal to develop standardized patient-reported and functional outcome measures to be used across clinical trials in NF.[51]
- Volumetric MRI analysis in conjunction with longitudinal imaging and clinical evaluation brought a greater understanding of the natural history of PN growth and of non-tumor manifestations in NF1 patients. Comparison of the characteristics of NF1 PN patients and pediatric cancer patients enrolling in clinical trials uncovered differences, which need to be considered in the design of NF1 PN trials.[52]
- In absence of a detailed knowledge of the natural history of PN, a randomized, placebo-controlled, flexible cross-over phase II study of tipifarnib in children and young adults with NF1 and PN provided early data on the natural history of PN while at the same time determining the activity of tipifarnib.[53] The placebo cohort was then used as a historical control group in subsequent treatment trials in PN utilizing identical eligibility criteria.[54-57]
- The development of a Department of Defense (DOD) sponsored Clinical Trials Consortium allowed acceleration of the conduct of multi-institutional trials for NF.[58]
- Development of mouse models of NF1 PN which recapitulate the disease resulted in preclinical testing and rational selections of agents to be evaluated in clinical trials.[59] For example, a mouse model of NF1 neurofibromas was predictive of the response to MEK inhibitors observed in a clinical trial.[60-62] A preclinical trials consortium sponsored by the Children’s Tumor Foundation (CTF) and the Neurofibromatosis Therapeutic Acceleration Program (NTAP) and promotes basic and translational discovery.
- Patient advocacy group involvement has contributed to patient education, fundraising and trial enrollment. CTF’s patient registry not only serves to gather information, but helps identify patients for clinical trials. CTF is also establishing a bio banking initiative.
- Regulatory agencies such as the FDA have contributed to clinical trial development in their willingness to discuss and consider alternative endpoints such as response by volumetric MRI, patient reported and functional outcomes. The support of NCI CTEP and of pharmaceutical companies to sponsor clinical trials for PN has been critical to the progress made.[53,55,63].